Human bullous pemphigoid antigen 2 transgenic skin elicits specific IgG in wild-type mice
- PMID: 17657247
- PMCID: PMC2546607
- DOI: 10.1038/sj.jid.5700970
Human bullous pemphigoid antigen 2 transgenic skin elicits specific IgG in wild-type mice
Abstract
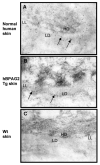
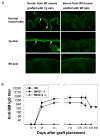
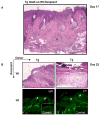
Bullous pemphigoid antigen 2 (BPAG2) is targeted by autoantibodies in patients with bullous pemphigoid (BP), and absent in patients with one type of epidermolysis bullosa (OMIM #226650). A keratin 14 promoter construct was used to produce transgenic (Tg) mice appropriately expressing human BPAG2 (hBPAG2) in murine epidermal basement membrane (BM). Grafts of Tg skin placed on gender-matched, syngeneic wild type (Wt) or major histocompatibility complex I (MHC I)-/- mice elicited IgG that bound human epidermal BM and BPAG2. Production of such IgG in grafted mice was prompt (detectable within 16+/-2 days), robust (titer > or = 1,280), durable (present > or = 380 days), and correlated with the involution and loss of Tg skin grafts. MHC II-/- mice grafted with Tg skin did not develop anti-hBPAG2 IgG or graft loss indicating that MHC II:CD4+ T cell interactions were crucial for these responses. Tg skin grafts on Wt mice developed neutrophil-rich infiltrates, dermal edema, subepidermal blisters, and deposits of immunoreactants in epidermal BM. This model shows fidelity to alterations seen in patients with BP, has relevance to immune responses that may arise in patients with epidermolysis bullosa following BPAG2 gene replacement, and can be used to identify interventions that may block production of IgG against proteins in epidermal BM.
Conflict of interest statement
Figures





Similar articles
-
Transient anti-CD40L co-stimulation blockade prevents immune responses against human bullous pemphigoid antigen 2: implications for gene therapy.J Invest Dermatol. 2009 May;129(5):1203-7. doi: 10.1038/jid.2008.364. Epub 2008 Nov 27. J Invest Dermatol. 2009. PMID: 19037236 Free PMC article.
-
Sequential intramolecular epitope spreading of humoral responses to human BPAG2 in a transgenic model.J Invest Dermatol. 2010 Apr;130(4):1040-7. doi: 10.1038/jid.2009.309. Epub 2009 Oct 8. J Invest Dermatol. 2010. PMID: 19812601
-
Transcutaneous gene gun delivery of hNC16A Induces BPAG2-specific tolerance.J Invest Dermatol. 2012 Jun;132(6):1665-71. doi: 10.1038/jid.2012.19. Epub 2012 Mar 1. J Invest Dermatol. 2012. PMID: 22377765
-
Bullous pemphigoid: What's ahead?J Dermatol. 2016 Mar;43(3):237-40. doi: 10.1111/1346-8138.13207. Epub 2015 Nov 25. J Dermatol. 2016. PMID: 26603373 Review.
-
Life before and beyond blistering: The role of collagen XVII in epidermal physiology.Exp Dermatol. 2019 Oct;28(10):1135-1141. doi: 10.1111/exd.13550. Epub 2018 Jun 28. Exp Dermatol. 2019. PMID: 29604146 Review.
Cited by
-
Development of an ELISA for sensitive and specific detection of IgA autoantibodies against BP180 in pemphigoid diseases.Orphanet J Rare Dis. 2011 May 28;6:31. doi: 10.1186/1750-1172-6-31. Orphanet J Rare Dis. 2011. PMID: 21619684 Free PMC article.
-
Mechanisms of Disease: Pemphigus and Bullous Pemphigoid.Annu Rev Pathol. 2016 May 23;11:175-97. doi: 10.1146/annurev-pathol-012615-044313. Epub 2016 Feb 22. Annu Rev Pathol. 2016. PMID: 26907530 Free PMC article. Review.
-
Immune Reaction to Type XVII Collagen Induces Intramolecular and Intermolecular Epitope Spreading in Experimental Bullous Pemphigoid Models.Front Immunol. 2019 Jun 19;10:1410. doi: 10.3389/fimmu.2019.01410. eCollection 2019. Front Immunol. 2019. PMID: 31275329 Free PMC article.
-
Transient anti-CD40L co-stimulation blockade prevents immune responses against human bullous pemphigoid antigen 2: implications for gene therapy.J Invest Dermatol. 2009 May;129(5):1203-7. doi: 10.1038/jid.2008.364. Epub 2008 Nov 27. J Invest Dermatol. 2009. PMID: 19037236 Free PMC article.
-
Distinct types of stem cell divisions determine organ regeneration and aging in hair follicles.Nat Aging. 2021 Feb;1(2):190-204. doi: 10.1038/s43587-021-00033-7. Epub 2021 Feb 11. Nat Aging. 2021. PMID: 37118636
References
-
- Darling TN, Bauer JW, Hintner H, Yancey KB. Generalized atrophic benign epidermolysis bullosa. Adv Dermatol. 1997a;13:87–119. - PubMed
-
- Darling TN, McGrath JA, Yee C, Gatalica B, Hametner R, Bauer JW, et al. Premature termination codons are present on both alleles of the bullous pemphigoid antigen 2/type XVII collagen gene in five Austrian families with generalized atrophic benign epidermolysis bullosa. J Invest Dermatol. 1997b;108:463–8. - PubMed
-
- Dumois JA, VanderVegt FP, Kopp JB, Marinos NJ, Rooney JF, Notkins AL. Transplantation of skin from human immunodeficiency virus type 1-transgenic mice to normal congenic mice results in graft rejection. J Infect Dis. 1995;172:232–4. - PubMed
-
- Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–62. - PubMed
-
- Franzke CW, Bruckner P, Bruckner-Tuderman L. Collagenous transmembrane proteins: recent insights into biology and pathology. J Biol Chem. 2005;280:4005–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

