Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo
- PMID: 17657311
- PMCID: PMC1924499
- DOI: 10.1172/JCI32057
Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo
Abstract
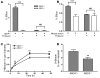
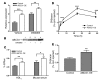
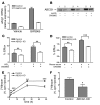
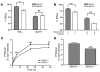
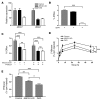
Macrophage ATP-binding cassette transporter A1 (ABCA1), scavenger receptor class B type I (SR-BI), and ABCG1 have been shown to promote cholesterol efflux to extracellular acceptors in vitro and influence atherosclerosis in mice, but their roles in mediating reverse cholesterol transport (RCT) from macrophages in vivo are unknown. Using an assay of macrophage RCT in mice, we found that primary macrophages lacking ABCA1 had a significant reduction in macrophage RCT in vivo, demonstrating the importance of ABCA1 in promoting macrophage RCT, however substantial residual RCT exists in the absence of macrophage ABCA1. Using primary macrophages deficient in SR-BI expression, we found that macrophage SR-BI, which was shown to promote cholesterol efflux in vitro, does not contribute to macrophage RCT in vivo. To investigate whether macrophage ABCG1 is involved in macrophage RCT in vivo, we used ABCG1-overexpressing, -knockdown, and -knockout macrophages. We show that increased macrophage ABCG1 expression significantly promoted while knockdown or knockout of macrophage ABCG1 expression significantly reduced macrophage RCT in vivo. Finally, we show that there was a greater decrease in macrophage RCT from cells where both ABCA1 and ABCG1 expression were knocked down than from ABCG1-knockdown cells. These results demonstrate that ABCA1 and ABCG1, but not SR-BI, promote macrophage RCT in vivo and are additive in their effects.
Figures
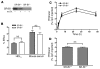
References
-
- Cuchel M., Rader D.J. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. - PubMed
-
- Linsel-Nitschke P., Tall A.R. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 2005;4:193–205. - PubMed
-
- Wang N., Silver D.L., Costet P., Tall A.R. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 2000;275:33053–33058. - PubMed
-
- Oram J.F., Lawn R.M., Garvin M.R., Wade D.P. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 2000;275:34508–34511. - PubMed
-
- Bortnick A.E., et al. The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J. Biol. Chem. 2000;275:28634–28640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

