Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury
- PMID: 17657312
- PMCID: PMC1924498
- DOI: 10.1172/JCI32041
Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury
Abstract
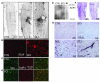
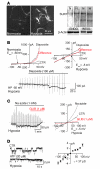
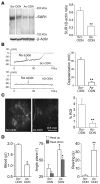
Acute spinal cord injury (SCI) causes progressive hemorrhagic necrosis (PHN), a poorly understood pathological process characterized by hemorrhage and necrosis that leads to devastating loss of spinal cord tissue, cystic cavitation of the cord, and debilitating neurological dysfunction. Using a rodent model of severe cervical SCI, we tested the hypothesis that sulfonylurea receptor 1-regulated (SUR1-regulated) Ca(2+)-activated, [ATP](i)-sensitive nonspecific cation (NC(Ca-ATP)) channels are involved in PHN. In control rats, SCI caused a progressively expansive lesion with fragmentation of capillaries, hemorrhage that doubled in volume over 12 hours, tissue necrosis, and severe neurological dysfunction. SUR1 expression was upregulated in capillaries and neurons surrounding necrotic lesions. Patch clamp of cultured endothelial cells exposed to hypoxia showed that upregulation of SUR1 was associated with expression of functional SUR1-regulated NC(Ca-ATP) channels. Following SCI, block of SUR1 by glibenclamide or repaglinide or suppression of Abcc8, which encodes for SUR1 by phosphorothioated antisense oligodeoxynucleotide essentially eliminated capillary fragmentation and progressive accumulation of blood, was associated with significant sparing of white matter tracts and a 3-fold reduction in lesion volume, and resulted in marked neurobehavioral functional improvement compared with controls. We conclude that SUR1-regulated NC(Ca-ATP) channels in capillary endothelium are critical to development of PHN and constitute a major target for therapy in SCI.
Figures


References
-
- Hayes K.C., Kakulas B.A. Neuropathology of human spinal cord injury sustained in sports-related activities. J. Neurotrauma. 1997;14:235–248. - PubMed
-
- Tator C.H., Fehlings M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 1991;75:15–26. - PubMed
-
- Kwon B.K., Tetzlaff W., Grauer J.N., Beiner J., Vaccaro A.R. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451–464. - PubMed
-
- Bilgen M., Abbe R., Liu S.J., Narayana P.A. Spatial and temporal evolution of hemorrhage in the hyperacute phase of experimental spinal cord injury: in vivo magnetic resonance imaging. Magn. Reson. Med. 2000;43:594–600. - PubMed
-
- Nelson E., Gertz S.D., Rennels M.L., Ducker T.B., Blaumanis O.R. Spinal cord injury. The role of vascular damage in the pathogenesis of central hemorrhagic necrosis. Arch. Neurol. 1977;34:332–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

