UNC-1 regulates gap junctions important to locomotion in C. elegans
- PMID: 17658257
- PMCID: PMC1976340
- DOI: 10.1016/j.cub.2007.06.060
UNC-1 regulates gap junctions important to locomotion in C. elegans
Abstract
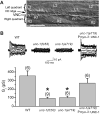
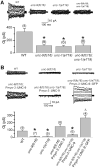
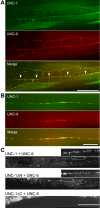
In C. elegans, loss-of-function (lf) mutations of the stomatin-like protein (SLP) UNC-1 and the innexin UNC-9 inhibit locomotion [1, 2] and modulate sensitivity to volatile anesthetics [3, 4]. It was unknown why unc-1(lf) and unc-9(lf) mutants have similar phenotypes. We tested the hypothesis that UNC-1 is a regulator of gap junctions formed by UNC-9. Analyses of junctional currents between body-wall muscle cells showed that electrical coupling was inhibited to a similar degree in unc-1(lf), unc-9(lf), and unc-1(lf);unc-9(lf) double mutants, suggesting that UNC-1 and UNC-9 function together. Expression of Punc-1::DsRED2 and Punc-9::GFP transcriptional fusions suggests that unc-1 and unc-9 are coexpressed in neurons and body-wall muscle cells. Immunohistochemistry showed that UNC-1 and UNC-9 colocalized at intercellular junctions and that unc-1(lf) did not alter UNC-9 expression or subcellular localization. Bimolecular fluorescence complementation (BiFC) assays suggest that UNC-1 and UNC-9 are physically very close at intercellular junctions. Targeted rescue experiments suggest that UNC-9 and UNC-1 function predominantly in neurons to control locomotion. Thus, in addition to the recently reported function of regulating mechanosensitive ion channels [5, 6], SLPs might have a novel function of regulating gap junctions.
Figures

Comment in
-
Innexin function: minding the gap junction.Curr Biol. 2007 Sep 18;17(18):R812-4. doi: 10.1016/j.cub.2007.07.043. Curr Biol. 2007. PMID: 17878053
References
-
- Sedensky MM, Meneely PM. Genetic analysis of halothane sensitivity in Caenorhabditis elegans. Science. 1987;236:952–954. - PubMed
-
- Goodman MB, Ernstrom GG, Chelur DS, O'Hagan R, Yao CA, Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

