Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice
- PMID: 17658499
- PMCID: PMC2731989
- DOI: 10.1016/j.cardiores.2007.06.024
Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice
Abstract
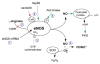
Nitric oxide (NO) is a gaseous molecule that plays many key roles in the cardiovascular system. Each of the enzymes that generate NO--neuronal, inducible and endothelial NO synthase-has been genetically disrupted in mice. This review discusses the cardiovascular phenotypes of each of the NO synthase (NOS) gene knockout mice, and the insights gained into the roles of NO in the cardiovascular system. Mice lacking the endothelial isoform are hypertensive, have endothelial dysfunction and show a more severe outcome in response to vascular injury, to stroke and cerebral ischaemia, and to diet-induced atherosclerosis. Mice lacking the neuronal isoform show a less severe outcome in response to stroke and cerebral ischaemia but have increased diet-induced atherosclerosis. Mice lacking the inducible isoform show reduced hypotension to septic shock. Together, NOS gene knockout mice have been useful tools that complement our other approaches to studying the multiple roles of NO in the cardiovascular system.
Conflict of interest statement
Conflict of interest: none declared.
Figures
References
-
- Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Ann Rev Biochem. 1994;63:175–195. - PubMed
-
- Radomski MW, Palmer RM, Moncada S. Modulation of platelet aggregation by an l-arginine-nitric oxide pathway. Trends Pharmacol Sci. 1991;12:87–88. - PubMed
-
- Freedman JE, Sauter R, Battinelli EM, Ault K, Knowles C, Huang PL, et al. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circ Res. 1999;84:1416–1421. - PubMed
-
- Lefer AM, Ma XL. Decreased basal nitric oxide release in hypercholesterolemia increases neutrophil adherence to rabbit coronary artery endothelium. Arterioscler Thromb. 1993;13:771–776. - PubMed

