Microfluidic modeling of cell-cell interactions in malaria pathogenesis
- PMID: 17658948
- PMCID: PMC1924869
- DOI: 10.1371/journal.ppat.0030099
Microfluidic modeling of cell-cell interactions in malaria pathogenesis
Abstract
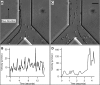
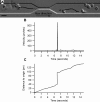
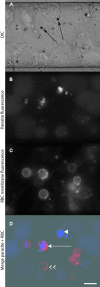
The clinical outcomes of human infections by Plasmodium falciparum remain highly unpredictable. A complete understanding of the complex interactions between host cells and the parasite will require in vitro experimental models that simultaneously capture diverse host-parasite interactions relevant to pathogenesis. Here we show that advanced microfluidic devices concurrently model (a) adhesion of infected red blood cells to host cell ligands, (b) rheological responses to changing dimensions of capillaries with shapes and sizes similar to small blood vessels, and (c) phagocytosis of infected erythrocytes by macrophages. All of this is accomplished under physiologically relevant flow conditions for up to 20 h. Using select examples, we demonstrate how this enabling technology can be applied in novel, integrated ways to dissect interactions between host cell ligands and parasitized erythrocytes in synthetic capillaries. The devices are cheap and portable and require small sample volumes; thus, they have the potential to be widely used in research laboratories and at field sites with access to fresh patient samples.
Conflict of interest statement
Figures

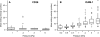

References
-
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. - PubMed
-
- Brown H, Hien TT, Day N, Mai NT, Chuong LV, et al. Evidence of blood–brain barrier dysfunction in human cerebral malaria. Neuropathol Appl Neurobiol. 1999;25:331–340. - PubMed
-
- Dondorp AM, Pongponratn E, White NJ. Reduced microcirculatory flow in severe falciparum malaria: Pathophysiology and electron-microscopic pathology. Acta Trop. 2004;89:309–317. - PubMed
-
- Cranston HA, Boylan CW, Carroll GL, Sutera SP, Williamson JR, et al. Plasmodium falciparum maturation abolishes physiologic red cell deformability. Science. 1984;223:400–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

