Prostaglandin F2-alpha receptor (FPr) expression on porcine corpus luteum microvascular endothelial cells (pCL-MVECs)
- PMID: 17659079
- PMCID: PMC1949401
- DOI: 10.1186/1477-7827-5-31
Prostaglandin F2-alpha receptor (FPr) expression on porcine corpus luteum microvascular endothelial cells (pCL-MVECs)
Abstract
Background: The corpus luteum (CL) is a transient endocrine gland and prostaglandin F2-alpha is considered to be the principal luteolysin in pigs. In this species, the in vivo administration of prostaglandin F2-alpha induces apoptosis in large vessels as early as 6 hours after administration. The presence of the prostaglandin F2-alpha receptor (FPr) on the microvascular endothelial cells (pCL-MVECs) of the porcine corpus luteum has not yet been defined. The aim of the study was to assess FPr expression in pCL-MVECs in the early and mid-luteal phases (EL-p, ML-p), and during pregnancy (P-p). Moreover, the effectiveness of prostaglandin F2-alpha treatment in inducing pCL-MVEC apoptosis was tested.
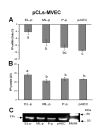
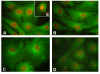
Methods: Porcine CLs were collected in the EL and ML phases and during P-p. All CLs from each animal were minced together and the homogenates underwent enzymatic digestion. The pCL-MVECs were then positively selected by an immunomagnetic separation protocol using Dynabeads coated with anti-CD31 monoclonal antibody and seeded in flasks in the presence of EGM 2-MV (Microvascular Endothelial Cell Medium-2). After 4 days of culture, the cells underwent additional immunomagnetic selection and were seeded in flasks until the confluent stage.PCR Real time, western blot and immunodetection assays were utilized to assess the presence of FPr on pCL-MVEC primary cultures. Furthermore, the influence of culture time (freshly isolated, cultured overnight and at confluence) and hormonal treatment (P4 and E2) on FPr expression in pCL-MVECs was also investigated. Apoptosis was detected by TUNEL assay of pCL-MVECs exposed to prostaglandin F2-alpha.
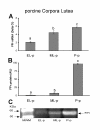
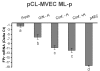
Results: We obtained primary cultures of pCL-MVECs from all animals. FPr mRNA and protein levels showed the highest value (ANOVA) in CL-MVECs derived from the early-luteal phase. Moreover, freshly isolated MVECs showed a higher FPr mRNA value than those cultured overnight and confluent cells (ANOVA). prostaglandin F2-alpha treatment failed to induce an apoptotic response in all the pCL-MVEC cultures.
Conclusion: Our data showing the presence of FPr on MVECs and the inability of prostaglandin F2-alpha to evoke an in vitro apoptotic response suggest that other molecules or mechanisms must be considered in order to explain the in vivo direct pro-apoptotic effect of prostaglandin F2-alpha at the endothelial level.
Figures


References
-
- Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. 2000;80:1–29. - PubMed
-
- Gadsby JE, Balapure AK, Britt JH, Fitz TA. Prostaglandin F2 alpha receptors on enzyme-dissociated pig luteal cells throughout the estrous cycle. Endocrinology. 1990;126:787–795. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

