Sublimation as a method of matrix application for mass spectrometric imaging
- PMID: 17659880
- PMCID: PMC2042488
- DOI: 10.1016/j.jasms.2007.06.010
Sublimation as a method of matrix application for mass spectrometric imaging
Abstract
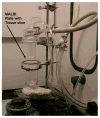
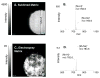
Common organic matrix-assisted laser desorption/ionization (MALDI) matrices, 2,5-dihydroxybenzoic acid, 3,5-dimethoxy-4-hydroxycinnamic acid, and alpha-cyano-4-hydroxycinnamic acid, were found to undergo sublimation without decomposition under conditions of reduced pressure and elevated temperature. This solid to vapor-phase transition was exploited to apply MALDI matrix onto tissue samples over a broad surface in a solvent-free application for mass spectrometric imaging. Sublimation of matrix produced an even layer of small crystals across the sample plate. The deposition was readily controlled with time, temperature, and pressure settings and was highly reproducible from one sample to the next. Mass spectrometric images acquired from phospholipid standards robotically spotted onto a MALDI plate yielded a more intense, even signal with fewer sodium adducts when matrix was applied by sublimation relative to samples where matrix was deposited by an electrospray technique. MALDI matrix could be readily applied to tissue sections on glass slides and stainless steel MALDI plate inserts as long as good thermal contact was made with the condenser of the sublimation device. Sections of mouse brain were coated with matrix applied by sublimation and were imaged using a Q-q-TOF mass spectrometer to yield mass spectral images of very high quality. Image quality is likely enhanced by several features of this technique including the microcrystalline morphology of the deposited matrix, increased purity of deposited matrix, and evenness of deposition. This inexpensive method was reproducible and eliminated the potential for spreading of analytes arising from solvent deposition during matrix application.
Figures



References
-
- Schwartz SA, Weil RJ, Johnson MD, Toms SA, Caprioli RM. Protein Profiling in Brain Tumors Using Mass Spectrometry: Feasibility of a New Technique for the Analysis of Protein Expression. Clin Cancer Res. 2004;10:981–987. - PubMed
-
- Khatib-Shahidi S, Andersson M, Herman JL, Gillespie TA, Caprioli RM. Direct Molecular Analysis of Whole-Body Animal Tissue Sections by Imaging MALDI Mass Spectrometry. Anal Chem. 2006;78:6448–6456. - PubMed
-
- Garrett TJ, Prieto-Conaway MC, Kovtoun V, Bui H, Izgarian N, Stafford G, Yost RA. Imaging of Small Molecules in Tissue Sections With a New Intermediate-Pressure MALDI Linear Ion Trap Mass Spectrometer. Intl J Mass Spectrom. 2007;260:166–176.
-
- Aerni HR, Cornett DS, Caprioli RM. Automated Acoustic Matrix Deposition for MALDI Sample Preparation. Anal Chem. 2006;78:827–834. - PubMed
-
- Jurchen JC, Rubakhin SS, Sweedler JV. MALDI-MS Imaging of Features Smaller Than the Size of the Laser Beam. J Am Soc Mass Spectrom. 2005;16:1654–1659. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

