Actions of anti-Mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition
- PMID: 17660231
- PMCID: PMC8260025
- DOI: 10.1530/REP-07-0119
Actions of anti-Mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition
Abstract
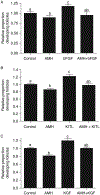
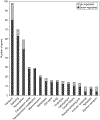
The oocytes found within the primordial follicles of mammalian ovaries remain quiescent for months to years until they receive the appropriate signals to undergo the primordial to primary follicle transition and initiate folliculogenesis. The molecular mechanisms and extracellular signaling factors that regulate this process remain to be fully elucidated. The current study investigates the mechanisms utilized by anti-Müllerian hormone (AMH; i.e. Müllerian inhibitory substance) to inhibit the primordial to primary follicle transition. Ovaries from 4-day-old rats were placed into organ culture and incubated in the absence or presence of AMH, either alone or in combination with known stimulators of follicle transition, including basic fibroblast growth factor (bFGF), kit ligand (KITL), or keratinocyte growth factor (KGF). Following 10 days of culture, the ovaries were sectioned, stained, and morphologically evaluated to determine the percentage of primordial versus developing follicles. As previously demonstrated, AMH treatment decreased primordial to primary follicle transition. Interestingly, AMH inhibited the stimulatory actions of KITL, bFGF, and KGF. Therefore, AMH can inhibit the basal and stimulated development of primordial follicles. To investigate the mechanism of AMH actions, the influence AMH has on the ovarian transcriptome was analyzed. AMH treatment when compared with controls was found to alter the expression of 707 genes. The overall effect of AMH exposure is to decrease the expression of stimulatory factors, increase the expression of inhibitory factors, and regulate cellular pathways (e.g. transforming growth factor beta signaling pathway) that result in the inhibition of primordial follicle development. Analysis of the regulatory factors and cellular pathways altered by AMH provides a better understanding of the molecular control of primordial follicle development.
Figures
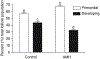



Similar articles
-
Keratinocyte growth factor acts as a mesenchymal factor that promotes ovarian primordial to primary follicle transition.Biol Reprod. 2005 Nov;73(5):967-73. doi: 10.1095/biolreprod.105.043117. Epub 2005 Jul 6. Biol Reprod. 2005. PMID: 16000551
-
Inhibitory actions of Anti-Müllerian Hormone (AMH) on ovarian primordial follicle assembly.PLoS One. 2011;6(5):e20087. doi: 10.1371/journal.pone.0020087. Epub 2011 May 27. PLoS One. 2011. PMID: 21637711 Free PMC article.
-
Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-Mullerian hormone production in the rat ovary.J Endocrinol. 2006 Dec;191(3):549-58. doi: 10.1677/joe.1.06592. J Endocrinol. 2006. PMID: 17170213
-
Regulation of primordial follicle assembly and development.Hum Reprod Update. 2005 Sep-Oct;11(5):461-71. doi: 10.1093/humupd/dmi020. Epub 2005 Jul 8. Hum Reprod Update. 2005. PMID: 16006439 Review.
-
A putative role for anti-Müllerian hormone (AMH) in optimising ovarian reserve expenditure.J Endocrinol. 2017 Apr;233(1):R1-R13. doi: 10.1530/JOE-16-0522. Epub 2017 Jan 27. J Endocrinol. 2017. PMID: 28130407 Review.
Cited by
-
Glial-derived neurotrophic factor promotes ovarian primordial follicle development and cell-cell interactions during folliculogenesis.Reproduction. 2008 May;135(5):671-82. doi: 10.1530/REP-07-0405. Epub 2008 Feb 27. Reproduction. 2008. PMID: 18304989 Free PMC article.
-
The Effect of AMH on Folliculogenesis.Reprod Sci. 2025 May 23. doi: 10.1007/s43032-025-01879-7. Online ahead of print. Reprod Sci. 2025. PMID: 40408029 Review.
-
New insights into anti-Müllerian hormone role in the hypothalamic-pituitary-gonadal axis and neuroendocrine development.Cell Mol Life Sci. 2021 Jan;78(1):1-16. doi: 10.1007/s00018-020-03576-x. Epub 2020 Jun 20. Cell Mol Life Sci. 2021. PMID: 32564094 Free PMC article. Review.
-
Single-cell sequencing reveals suppressive transcriptional programs regulated by MIS/AMH in neonatal ovaries.Proc Natl Acad Sci U S A. 2021 May 18;118(20):e2100920118. doi: 10.1073/pnas.2100920118. Proc Natl Acad Sci U S A. 2021. PMID: 33980714 Free PMC article.
-
Interactions between serum FSH, inhibin B and antral follicle count in the decline of serum AMH during the menstrual cycle in late reproductive age.Endocrinol Diabetes Metab. 2020 Aug 8;4(2):e00172. doi: 10.1002/edm2.172. eCollection 2021 Apr. Endocrinol Diabetes Metab. 2020. PMID: 33855196 Free PMC article.
References
-
- Arici A, Oral E, Bahtiyar O, Engin O, Seli E & Jones E 1997. Leukaemia inhibitory factor expression in human follicular fluid and ovarian cells. Human Reproduction 12 1233–1239. - PubMed
-
- Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D & Harper SJ 2002. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Research 62 4123–4131. - PubMed
-
- Ben-Haroush A, Abir R, Ao A, Jin S, Kessler-Icekson G, Feldberg D & Fisch B 2005. Expression of basic fibroblast growth factor and its receptors in human ovarian follicles from adults and fetuses. Fertility and Sterility 84 1257–1268. - PubMed
-
- Bove SE, Petroff MG, Nishibori M & Pate JL 2000. Macrophage migration inhibitory factor in the bovine corpus luteum: characterization of steady-state messenger ribonucleic acid and immunohistochemical localization. Biology of Reproduction 62 879–885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

