Docking and homology modeling explain inhibition of the human vesicular glutamate transporters
- PMID: 17660252
- PMCID: PMC2206968
- DOI: 10.1110/ps.072944707
Docking and homology modeling explain inhibition of the human vesicular glutamate transporters
Abstract
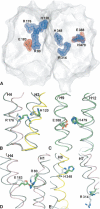
As membrane transporter proteins, VGLUT1-3 mediate the uptake of glutamate into synaptic vesicles at presynaptic nerve terminals of excitatory neural cells. This function is crucial for exocytosis and the role of glutamate as the major excitatory neurotransmitter in the central nervous system. The three transporters, sharing 76% amino acid sequence identity in humans, are highly homologous but differ in regional expression in the brain. Although little is known regarding their three-dimensional structures, hydropathy analysis on these proteins predicts 12 transmembrane segments connected by loops, a topology similar to other members in the major facilitator superfamily, where VGLUT1-3 have been phylogenetically classified. In this work, we present a three-dimensional model for the human VGLUT1 protein based on its distant bacterial homolog in the same superfamily, the glycerol-3-phosphate transporter from Escherichia coli. This structural model, stable during molecular dynamics simulations in phospholipid bilayers solvated by water, reveals amino acid residues that face its pore and are likely to affect substrate translocation. Docking of VGLUT1 substrates to this pore localizes two different binding sites, to which inhibitors also bind with an overall trend in binding affinity that is in agreement with previously published experimental data.
Figures



Similar articles
-
Interaction between the vesicular glutamate transporter type 1 and endophilin A1, a protein essential for endocytosis.J Neurochem. 2006 May;97(4):1111-25. doi: 10.1111/j.1471-4159.2006.03821.x. Epub 2006 Apr 5. J Neurochem. 2006. PMID: 16606361
-
An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size.Proc Natl Acad Sci U S A. 2004 May 4;101(18):7158-63. doi: 10.1073/pnas.0401764101. Epub 2004 Apr 21. Proc Natl Acad Sci U S A. 2004. PMID: 15103023 Free PMC article.
-
Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia.Synapse. 2003 Nov;50(2):117-29. doi: 10.1002/syn.10249. Synapse. 2003. PMID: 12923814
-
Structure-Function Relationship of Transporters in the Glutamate-Glutamine Cycle of the Central Nervous System.Int J Mol Sci. 2018 Apr 12;19(4):1177. doi: 10.3390/ijms19041177. Int J Mol Sci. 2018. PMID: 29649168 Free PMC article. Review.
-
VGLUT substrates and inhibitors: A computational viewpoint.Biochim Biophys Acta Biomembr. 2020 Dec 1;1862(12):183175. doi: 10.1016/j.bbamem.2020.183175. Epub 2020 Jan 7. Biochim Biophys Acta Biomembr. 2020. PMID: 31923412 Free PMC article. Review.
Cited by
-
Screening of the SLC17A8 gene as a causative factor for autosomal dominant non-syndromic hearing loss in Koreans.BMC Med Genet. 2016 Jan 22;17:6. doi: 10.1186/s12881-016-0269-3. BMC Med Genet. 2016. PMID: 26797701 Free PMC article.
-
From glutamate co-release to vesicular synergy: vesicular glutamate transporters.Nat Rev Neurosci. 2011 Apr;12(4):204-16. doi: 10.1038/nrn2969. Nat Rev Neurosci. 2011. PMID: 21415847 Review.
-
Protons Regulate Vesicular Glutamate Transporters through an Allosteric Mechanism.Neuron. 2016 May 18;90(4):768-80. doi: 10.1016/j.neuron.2016.03.026. Epub 2016 Apr 28. Neuron. 2016. PMID: 27133463 Free PMC article.
-
Structure-function studies of the SLC17 transporter sialin identify crucial residues and substrate-induced conformational changes.J Biol Chem. 2010 Jun 18;285(25):19316-23. doi: 10.1074/jbc.M110.130716. Epub 2010 Apr 27. J Biol Chem. 2010. PMID: 20424173 Free PMC article.
-
The dual role of chloride in synaptic vesicle glutamate transport.Elife. 2018 Jul 24;7:e34896. doi: 10.7554/eLife.34896. Elife. 2018. PMID: 30040066 Free PMC article.
References
-
- Abramson J., Smirnova, I., Kasho, V., Verner, G., Kaback, H.R., and Iwata, S. 2003a. Structure and mechanism of the lactose permease of Escherichia coli . Science 301 610–615. - PubMed
-
- Abramson J., Smirnova, I., Kasho, V., Verner, G., and Iwata, S. 2003b. The lactose permease of Escherichia coli: Overall structure, the sugar-binding site, and the alternating access model for transport. FEBS Lett. 555 96–101. - PubMed
-
- Aihara Y., Mashima, H., Onda, H., Hisano, S., Kasuya, H., Hori, T., Yamada, S., Tomura, H., Yamada, Y., Inoue, I., et al. 2000. Molecular cloning of a novel brain-type Na-dependent inorganic phosphate cotransporter. J. Neurochem. 74 2622–2625. - PubMed
-
- Almqvist J., Huang, Y., Hovmoller, S., and Wang, D.N. 2004. Homology modeling of the human microsomal glucose 6-phosphate transporter explains the mutations that cause the glycogen storage disease type Ib. Biochemistry 43 9289–9297. - PubMed
-
- Bellocchio E.E., Reimer, R.J., Fremeau Jr, R.T., and Edwards, R.H. 2000. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science 289 957–960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

