Branching in the sequential folding pathway of cytochrome c
- PMID: 17660254
- PMCID: PMC2206985
- DOI: 10.1110/ps.072922307
Branching in the sequential folding pathway of cytochrome c
Abstract
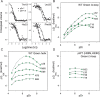
Previous results indicate that the folding pathways of cytochrome c and other proteins progressively build the target native protein in a predetermined stepwise manner by the sequential formation and association of native-like foldon units. The present work used native state hydrogen exchange methods to investigate a structural anomaly in cytochrome c results that suggested the concerted folding of two segments that have little structural relationship in the native protein. The results show that the two segments, an 18-residue omega loop and a 10-residue helix, are able to unfold and refold independently, which allows a branch point in the folding pathway. The pathway that emerges assembles native-like foldon units in a linear sequential manner when prior native-like structure can template a single subsequent foldon, and optional pathway branching is seen when prior structure is able to support the folding of two different foldons.
Figures
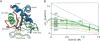
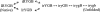

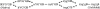

Similar articles
-
Protein folding: the stepwise assembly of foldon units.Proc Natl Acad Sci U S A. 2005 Mar 29;102(13):4741-6. doi: 10.1073/pnas.0501043102. Epub 2005 Mar 17. Proc Natl Acad Sci U S A. 2005. PMID: 15774579 Free PMC article.
-
Order of steps in the cytochrome C folding pathway: evidence for a sequential stabilization mechanism.J Mol Biol. 2006 Jun 23;359(5):1410-9. doi: 10.1016/j.jmb.2006.04.035. Epub 2006 May 2. J Mol Biol. 2006. PMID: 16690080
-
Functional role of a protein foldon--an Omega-loop foldon controls the alkaline transition in ferricytochrome c.Proteins. 2006 May 1;63(2):349-55. doi: 10.1002/prot.20757. Proteins. 2006. PMID: 16287119
-
Protein folding and misfolding: mechanism and principles.Q Rev Biophys. 2007 Nov;40(4):287-326. doi: 10.1017/S0033583508004654. Epub 2008 Apr 14. Q Rev Biophys. 2007. PMID: 18405419 Free PMC article. Review.
-
The nature of protein folding pathways.Proc Natl Acad Sci U S A. 2014 Nov 11;111(45):15873-80. doi: 10.1073/pnas.1411798111. Epub 2014 Oct 17. Proc Natl Acad Sci U S A. 2014. PMID: 25326421 Free PMC article. Review.
Cited by
-
Kinetic barriers and the role of topology in protein and RNA folding.Protein Sci. 2008 Aug;17(8):1308-18. doi: 10.1110/ps.036319.108. Epub 2008 May 23. Protein Sci. 2008. PMID: 18502978 Free PMC article. Review.
-
Becoming a peroxidase: cardiolipin-induced unfolding of cytochrome c.J Phys Chem B. 2013 Oct 24;117(42):12878-86. doi: 10.1021/jp402104r. Epub 2013 Jun 25. J Phys Chem B. 2013. PMID: 23713573 Free PMC article.
-
Mapping polymerization and allostery of hemoglobin S using point mutations.J Phys Chem B. 2013 Oct 24;117(42):13058-68. doi: 10.1021/jp4025156. Epub 2013 Sep 9. J Phys Chem B. 2013. PMID: 23957820 Free PMC article.
-
Genetically Encoded Fluorescent Probe for Detection of Heme-Induced Conformational Changes in Cytochrome c.Biosensors (Basel). 2023 Sep 18;13(9):890. doi: 10.3390/bios13090890. Biosensors (Basel). 2023. PMID: 37754124 Free PMC article.
-
Quantifying the structural requirements of the folding transition state of protein A and other systems.J Mol Biol. 2008 Sep 19;381(5):1362-81. doi: 10.1016/j.jmb.2008.06.067. Epub 2008 Jul 1. J Mol Biol. 2008. PMID: 18625237 Free PMC article.
References
-
- Bai Y. and Englander, S.W. 1996. Future directions in folding: The multi-state nature of protein structure. Proteins 24 145–151. - PubMed
-
- Baldwin R.L. 1995. The nature of protein folding pathways: The classical versus the new view. J. Biomol. NMR 5 103–109. - PubMed
-
- Bieri O., Wildegger, G., Bachmann, A., Wagner, C., and Kiefhaber, T. 1999. A salt-induced kinetic intermediate is on a new parallel pathway of lysozyme folding. Biochemistry 38 12460–12470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

