Molecular plasticity of beta-catenin: new insights from single-molecule measurements and MD simulation
- PMID: 17660262
- PMCID: PMC2206973
- DOI: 10.1110/ps.072773007
Molecular plasticity of beta-catenin: new insights from single-molecule measurements and MD simulation
Abstract
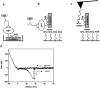
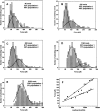
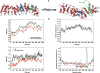
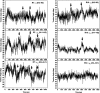
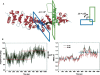
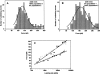
The multifunctional protein, beta-catenin, has essential roles in cell adhesion and, through the Wnt signaling pathway, in controlling cell differentiation, development, and generation of cancer. Could distinct molecular forms of beta-catenin underlie these two functions? Our single-molecule force spectroscopy of armadillo beta-catenin, with molecular dynamics (MD) simulation, suggests a model in which the cell generates various forms of beta-catenin, in equilibrium. We find beta-catenin and the transcriptional factor Tcf4 form two complexes with different affinities. Specific cellular response is achieved by the ligand binding to a particular matching preexisting conformer. Our MD simulation indicates that complexes derive from two conformers of the core region of the protein, whose preexisting molecular forms could arise from small variations in flexible regions of the beta-catenin main binding site. This mechanism for the generation of the various forms offers a route to tailoring future therapeutic strategies.
Figures

References
-
- Behrens J. and Lustig, B. 2004. The Wnt connection to tumorigenesis. Int. J. Dev. Biol. 48 477–487. - PubMed
-
- Behrens J., von Kries, J.P., Kuhl, M., Bruhn, L., Wedlich, D., Grosschedl, R., and Birchmeier, W. 1996. Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382 638–642. - PubMed
-
- Case D.L., Darden, T.A., Cheatham, T.E., Simmerling, C.L., Wang, J., Duke, R.E., Luo, R., Merz, K.M., Wang, B., Pearlman, D.A., et al. 2004. AMBER 8. University of California, San Francisco, CA.
-
- Changeux J.P. and Edelstein, S.J. 2005. Allosteric mechanisms of signal transduction. Science 308 1424–1428. - PubMed
-
- Choi H.J., Huber, A.H., and Weis, W.I. 2006. Thermodynamics of β-catenin–ligand interactions: The roles of the N- and C-terminal tails in modulating binding affinity. J. Biol. Chem. 281 1027–1038. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

