Docking of the periplasmic FecB binding protein to the FecCD transmembrane proteins in the ferric citrate transport system of Escherichia coli
- PMID: 17660286
- PMCID: PMC2045206
- DOI: 10.1128/JB.00884-07
Docking of the periplasmic FecB binding protein to the FecCD transmembrane proteins in the ferric citrate transport system of Escherichia coli
Abstract
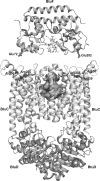
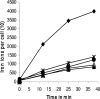
Citrate-mediated iron transport across the cytoplasmic membrane is catalyzed by an ABC transporter that consists of the periplasmic binding protein FecB, the transmembrane proteins FecC and FecD, and the ATPase FecE. Salt bridges between glutamate residues of the binding protein and arginine residues of the transmembrane proteins are predicted to mediate the positioning of the substrate-loaded binding protein on the transmembrane protein, based on the crystal structures of the ABC transporter for vitamin B(12), consisting of the BtuF binding protein and the BtuCD transmembrane proteins (E. L. Borths et al., Proc. Natl. Acad. Sci. USA 99:16642-16647, 2002). Here, we examined the role of the residues predicted to be involved in salt-bridge formation between FecB and FecCD by substituting these residues with alanine, cysteine, arginine, and glutamate and by analyzing the citrate-mediated iron transport of the mutants. Replacement of E93 in FecB with alanine [FecB(E93A)], cysteine, or arginine nearly abolished citrate-mediated iron transport. Mutation FecB(E222R) nearly eliminated transport, and FecB(E222A) and FecB(E222C) strongly reduced transport. FecD(R54C) and FecD(R51E) abolished transport, whereas other R-to-C mutations in putative interaction sites between FecCD and FecB substantially reduced transport. The introduced cysteine residues in FecB and FecCD also served to examine the formation of disulfide bridges in place of salt bridges between the binding protein and the transmembrane proteins. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis results suggest cross-linking of FecB(E93C) to FecD(R54C) and FecB(E222C) to FecC(R60C). The data are consistent with the proposal that FecB(E93) is contained in the region that binds to FecD and FecB(E222) in the region that binds to FecC.
Figures

References
-
- Angerer, A., and V. Braun. 1998. Iron regulates transcription of the Escherichia coli ferric citrate transport genes directly and through the transcription initiation proteins. Arch. Microbiol. 169:483-490. - PubMed
-
- Braun, V. 1997. Surface signaling: novel transcription initiation mechanism starting from the cell surface. Arch. Microbiol. 167:325-331. - PubMed
-
- Braun, V., S. Mahren, and M. Ogierman. 2003. Regulation of the FecI-type ECF sigma factor by transmembrane signalling. Curr. Opin. Microbiol. 6:173-180. - PubMed
-
- Braun, V., and S. Mahren. 2005. Transmembrane transcriptional control (surface signalling) of the Escherichia coli Fec type FEMS Microbiol. Rev. 29:673-684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

