Salicylic acid, yersiniabactin, and pyoverdin production by the model phytopathogen Pseudomonas syringae pv. tomato DC3000: synthesis, regulation, and impact on tomato and Arabidopsis host plants
- PMID: 17660289
- PMCID: PMC2045226
- DOI: 10.1128/JB.00827-07
Salicylic acid, yersiniabactin, and pyoverdin production by the model phytopathogen Pseudomonas syringae pv. tomato DC3000: synthesis, regulation, and impact on tomato and Arabidopsis host plants
Abstract
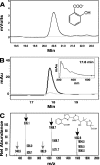
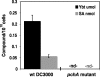
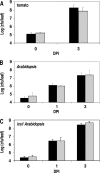
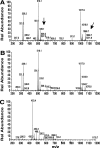
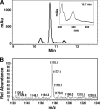
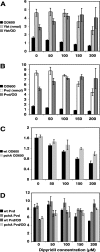
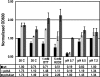
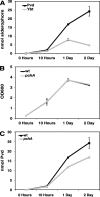
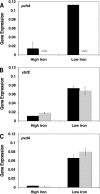
A genetically tractable model plant pathosystem, Pseudomonas syringae pv. tomato DC3000 on tomato and Arabidopsis thaliana hosts, was used to investigate the role of salicylic acid (SA) and iron acquisition via siderophores in bacterial virulence. Pathogen-induced SA accumulation mediates defense in these plants, and DC3000 contains the genes required for the synthesis of SA, the SA-incorporated siderophore yersiniabactin (Ybt), and the fluorescent siderophore pyoverdin (Pvd). We found that DC3000 synthesizes SA, Ybt, and Pvd under iron-limiting conditions in culture. Synthesis of SA and Ybt by DC3000 requires pchA, an isochorismate synthase gene in the Ybt genomic cluster, and exogenous SA can restore Ybt production by the pchA mutant. Ybt was also produced by DC3000 in planta, suggesting that Ybt plays a role in DC3000 pathogenesis. However, the pchA mutant did not exhibit any growth defect or altered virulence in plants. This lack of phenotype was not attributable to plant-produced SA restoring Ybt production, as the pchA mutant grew similarly to DC3000 in an Arabidopsis SA biosynthetic mutant, and in planta Ybt was not detected in pchA-infected wild-type plants. In culture, no growth defect was observed for the pchA mutant versus DC3000 for any condition tested. Instead, enhanced growth of the pchA mutant was observed under stringent iron limitation and additional stresses. This suggests that SA and Ybt production by DC3000 is costly and that Pvd is sufficient for iron acquisition. Further exploration of the comparative synthesis and utility of Ybt versus Pvd production by DC3000 found siderophore-dependent amplification of ybt gene expression to be absent, suggesting that Ybt may play a yet unknown role in DC3000 pathogenesis.
Figures
Similar articles
-
The phytopathogen Pseudomonas syringae pv. tomato DC3000 has three high-affinity iron-scavenging systems functional under iron limitation conditions but dispensable for pathogenesis.J Bacteriol. 2011 Jun;193(11):2767-75. doi: 10.1128/JB.00069-10. Epub 2011 Mar 25. J Bacteriol. 2011. PMID: 21441525 Free PMC article.
-
The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000.Mol Plant Microbe Interact. 2007 Aug;20(8):955-65. doi: 10.1094/MPMI-20-8-0955. Mol Plant Microbe Interact. 2007. PMID: 17722699
-
Identification of a twin-arginine translocation system in Pseudomonas syringae pv. tomato DC3000 and its contribution to pathogenicity and fitness.J Bacteriol. 2005 Dec;187(24):8450-61. doi: 10.1128/JB.187.24.8450-8461.2005. J Bacteriol. 2005. PMID: 16321949 Free PMC article.
-
Defining essential processes in plant pathogenesis with Pseudomonas syringae pv. tomato DC3000 disarmed polymutants and a subset of key type III effectors.Mol Plant Pathol. 2018 Jul;19(7):1779-1794. doi: 10.1111/mpp.12655. Epub 2018 Feb 1. Mol Plant Pathol. 2018. PMID: 29277959 Free PMC article. Review.
-
Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria.Biomolecules. 2021 May 9;11(5):705. doi: 10.3390/biom11050705. Biomolecules. 2021. PMID: 34065121 Free PMC article. Review.
Cited by
-
Iron acquisition strategies in pseudomonads: mechanisms, ecology, and evolution.Biometals. 2023 Aug;36(4):777-797. doi: 10.1007/s10534-022-00480-8. Epub 2022 Dec 12. Biometals. 2023. PMID: 36508064 Free PMC article. Review.
-
Impact of siderophore production by Pseudomonas syringae pv. syringae 22d/93 on epiphytic fitness and biocontrol activity against Pseudomonas syringae pv. glycinea 1a/96.Appl Environ Microbiol. 2010 May;76(9):2704-11. doi: 10.1128/AEM.02979-09. Epub 2010 Mar 5. Appl Environ Microbiol. 2010. PMID: 20208028 Free PMC article.
-
Characterization of pyoverdine and achromobactin in Pseudomonas syringae pv. phaseolicola 1448a.BMC Microbiol. 2011 Oct 3;11:218. doi: 10.1186/1471-2180-11-218. BMC Microbiol. 2011. PMID: 21967163 Free PMC article.
-
Role of the FeoB protein and siderophore in promoting virulence of Xanthomonas oryzae pv. oryzae on rice.J Bacteriol. 2010 Jun;192(12):3187-203. doi: 10.1128/JB.01558-09. Epub 2010 Apr 9. J Bacteriol. 2010. PMID: 20382771 Free PMC article.
-
Proteobactin and a yersiniabactin-related siderophore mediate iron acquisition in Proteus mirabilis.Mol Microbiol. 2010 Oct;78(1):138-57. doi: 10.1111/j.1365-2958.2010.07317.x. Mol Microbiol. 2010. PMID: 20923418 Free PMC article.
References
-
- Alekshun, M. N., and S. B. Levy. 1999. The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 7:410-413. - PubMed
-
- Alonso, J. M., and J. R. Ecker. 2006. Moving forward in reverse: genetic technologies to enable genome-wide phenomic screens in Arabidopsis. Nat. Rev. Genet. 7:524-536. - PubMed
-
- Anisimov, R., D. Brem, J. Heesemann, and A. Rakin. 2005. Molecular mechanism of YbtA-mediated transcriptional regulation of divergent overlapping promoters ybtA and irp6 of Yersinia enterocolitica. FEMS Microbiol. Lett. 250:27-32. - PubMed
-
- Audenaert, K., T. Pattery, P. Cornelis, and M. Hofte. 2002. Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: role of salicylic acid, pyochelin, and pyocyanin. Mol. Plant-Microbe Interact. 15:1147-1156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

