Mechanism of islet amyloid polypeptide fibrillation at lipid interfaces studied by infrared reflection absorption spectroscopy
- PMID: 17660321
- PMCID: PMC2025658
- DOI: 10.1529/biophysj.107.110635
Mechanism of islet amyloid polypeptide fibrillation at lipid interfaces studied by infrared reflection absorption spectroscopy
Abstract
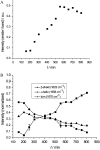
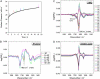
Islet amyloid polypeptide (IAPP) is a pancreatic hormone and one of a number of proteins that are involved in the formation of amyloid deposits in the islets of Langerhans of type II diabetes mellitus patients. Though IAPP-membrane interactions are known to play a major role in the fibrillation process, the mechanism and the peptide's conformational changes involved are still largely unknown. To obtain new insights into the conformational dynamics of IAPP upon its aggregation at membrane interfaces and to relate these structures to its fibril formation, we studied the association of IAPP at various interfaces including neutral as well as charged phospholipids using infrared reflection absorption spectroscopy. The results obtained reveal that the interaction of human IAPP with the lipid interface is driven by the N-terminal part of the peptide and is largely driven by electrostatic interactions, as the protein is able to associate strongly with negatively charged lipids only. A two-step process is observed upon peptide binding, involving a conformational transition from a largely alpha-helical to a beta-sheet conformation, finally forming ordered fibrillar structures. As revealed by simulations of the infrared reflection absorption spectra and complementary atomic force microscopy studies, the fibrillar structures formed consist of parallel intermolecular beta-sheets lying parallel to the lipid interface but still contain a significant number of turn structures. We may assume that these dynamical conformational changes observed for negatively charged lipid interfaces play an important role as the first steps of IAPP-induced membrane damage in type II diabetes.
Figures



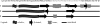


References
-
- Scheibel, T. 2005. Protein fibers as performance proteins: new technologies and applications. Curr. Opin. Biotechnol. 16:427–433. - PubMed
-
- Kelly, J. W. 1998. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathway. Curr. Opin. Struct. Biol. 8:101–106. - PubMed
-
- Goedert, M. 2001. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2:492–501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

