Light-independent phytochrome signaling mediated by dominant GAF domain tyrosine mutants of Arabidopsis phytochromes in transgenic plants
- PMID: 17660358
- PMCID: PMC1955707
- DOI: 10.1105/tpc.107.051516
Light-independent phytochrome signaling mediated by dominant GAF domain tyrosine mutants of Arabidopsis phytochromes in transgenic plants
Erratum in
- Plant Cell. 2007 Aug;19(8):2693-4
Abstract
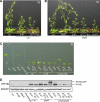
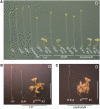
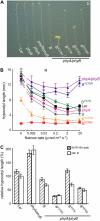
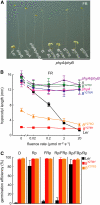
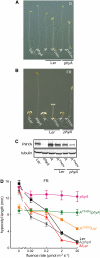
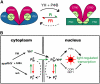
The photoreversibility of plant phytochromes enables continuous surveillance of the ambient light environment. Through expression of profluorescent, photoinsensitive Tyr-to-His mutant alleles of Arabidopsis thaliana phytochrome B (PHYB(Y276H)) and Arabidopsis phytochrome A (PHYA(Y242H)) in transgenic Arabidopsis plants, we demonstrate that photoconversion is not a prerequisite for phytochrome signaling. PHYB(Y276H)-expressing plants exhibit chromophore-dependent constitutive photomorphogenesis, light-independent phyB(Y276H) nuclear localization, constitutive activation of genes normally repressed in darkness, and light-insensitive seed germination. Fluence rate analyses of transgenic plants expressing PHYB(Y276H), PHYA(Y242H), and other Y(GAF) mutant alleles of PHYB demonstrate that a range of altered light-signaling activities are associated with mutation of this residue. We conclude that the universally conserved GAF domain Tyr residue, with which the bilin chromophore is intimately associated, performs a critical role in coupling light perception to signal transduction by plant phytochromes.
Figures



Similar articles
-
A non-covalently attached chromophore can mediate phytochrome B signaling in Arabidopsis.Plant Cell Physiol. 2011 Dec;52(12):2088-102. doi: 10.1093/pcp/pcr139. Epub 2011 Oct 17. Plant Cell Physiol. 2011. PMID: 22006939
-
Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana.Proc Natl Acad Sci U S A. 1996 Jul 23;93(15):8129-33. doi: 10.1073/pnas.93.15.8129. Proc Natl Acad Sci U S A. 1996. PMID: 8755615 Free PMC article.
-
Fluence and wavelength requirements for Arabidopsis CAB gene induction by different phytochromes.Plant Physiol. 1997 Dec;115(4):1533-40. doi: 10.1104/pp.115.4.1533. Plant Physiol. 1997. PMID: 9414562 Free PMC article.
-
Phytochrome signaling mechanisms and the control of plant development.Trends Cell Biol. 2011 Nov;21(11):664-71. doi: 10.1016/j.tcb.2011.07.002. Epub 2011 Aug 17. Trends Cell Biol. 2011. PMID: 21852137 Free PMC article. Review.
-
Mathematical models light up plant signaling.Plant Cell. 2014 Jan;26(1):5-20. doi: 10.1105/tpc.113.120006. Epub 2014 Jan 30. Plant Cell. 2014. PMID: 24481073 Free PMC article. Review.
Cited by
-
Structure-guided engineering of plant phytochrome B with altered photochemistry and light signaling.Plant Physiol. 2013 Mar;161(3):1445-57. doi: 10.1104/pp.112.208892. Epub 2013 Jan 15. Plant Physiol. 2013. PMID: 23321421 Free PMC article.
-
Phytochrome Signaling Is Mediated by PHYTOCHROME INTERACTING FACTOR in the Liverwort Marchantia polymorpha.Plant Cell. 2016 Jun;28(6):1406-21. doi: 10.1105/tpc.15.01063. Epub 2016 Jun 1. Plant Cell. 2016. PMID: 27252292 Free PMC article.
-
GAF domain is essential for nitrate-dependent AtNLP7 function.BMC Plant Biol. 2022 Jul 25;22(1):366. doi: 10.1186/s12870-022-03755-x. BMC Plant Biol. 2022. PMID: 35871642 Free PMC article.
-
The heat response regulators HSFA1s promote Arabidopsis thermomorphogenesis via stabilizing PIF4 during the day.Sci Adv. 2023 Nov 3;9(44):eadh1738. doi: 10.1126/sciadv.adh1738. Epub 2023 Nov 3. Sci Adv. 2023. PMID: 37922351 Free PMC article.
-
LOF and GOF Alleles Shed Light on the Molecular Basis of phyB Signaling in Plants.Plant Cell. 2019 Jul;31(7):1400-1401. doi: 10.1105/tpc.19.00373. Epub 2019 May 13. Plant Cell. 2019. PMID: 31085578 Free PMC article. No abstract available.
References
-
- Abe, T., Thitamadee, S., and Hashimoto, T. (2004). Microtubule defects and cell morphogenesis in the lefty1lefty2 tubulin mutant of Arabidopsis thaliana. Plant Cell Physiol. 45 211–220. - PubMed
-
- Al-Sady, B., Ni, W.M., Kircher, S., Schafer, E., and Quail, P.H. (2006). Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasorne-mediated degradation. Mol. Cell 23 439–446. - PubMed
-
- Batschauer, A., Rocholl, M., Kaiser, T., Nagatani, A., Furuya, M., and Schäfer, E. (1996). Blue and UVA light-regulated CHS expression in Arabidopsis independent of phytochrome A and phytochrome B. Plant J. 9 63–69.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

