Meiotic pairing and disjunction of mini-X chromosomes in drosophila is mediated by 240-bp rDNA repeats and the homolog conjunction proteins SNM and MNM
- PMID: 17660566
- PMCID: PMC2034643
- DOI: 10.1534/genetics.107.073866
Meiotic pairing and disjunction of mini-X chromosomes in drosophila is mediated by 240-bp rDNA repeats and the homolog conjunction proteins SNM and MNM
Abstract
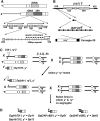
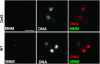
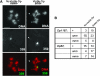
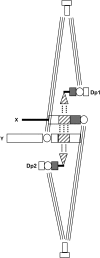
In most eukaryotes, segregation of homologous chromosomes during meiosis is dependent on crossovers that occur while the homologs are intimately paired during early prophase. Crossovers generate homolog connectors known as chiasmata that are stabilized by cohesion between sister-chromatid arms. In Drosophila males, homologs pair and segregate without recombining or forming chiasmata. Stable pairing of homologs is dependent on two proteins, SNM and MNM, that associate with chromosomes throughout meiosis I until their removal at anaphase I. SNM and MNM localize to the rDNA region of the X-Y pair, which contains 240-bp repeats that have previously been shown to function as cis-acting chromosome pairing/segregation sites. Here we show that heterochromatic mini-X chromosomes lacking native rDNA but carrying transgenic 240-bp repeat arrays segregate preferentially from full-length sex chromosomes and from each other. Mini-X pairs do not form autonomous bivalents but do associate at high frequency with the X-Y bivalent to form trivalents and quadrivalents. Both disjunction of mini-X pairs and multivalent formation are dependent on the presence of SNM and MNM. These results imply that 240-bp repeats function to mediate association of sex chromosomes with SNM and MNM.
Figures

Similar articles
-
Identification of two proteins required for conjunction and regular segregation of achiasmate homologs in Drosophila male meiosis.Cell. 2005 Nov 18;123(4):555-68. doi: 10.1016/j.cell.2005.08.043. Cell. 2005. PMID: 16286005
-
MNM and SNM maintain but do not establish achiasmate homolog conjunction during Drosophila male meiosis.PLoS Genet. 2019 May 28;15(5):e1008162. doi: 10.1371/journal.pgen.1008162. eCollection 2019 May. PLoS Genet. 2019. PMID: 31136586 Free PMC article.
-
Role of the mod(mdg4) common region in homolog segregation in Drosophila male meiosis.Genetics. 2007 May;176(1):161-80. doi: 10.1534/genetics.106.063289. Epub 2007 Feb 4. Genetics. 2007. PMID: 17277376 Free PMC article.
-
Homolog pairing and segregation in Drosophila meiosis.Genome Dyn. 2009;5:56-68. doi: 10.1159/000166619. Genome Dyn. 2009. PMID: 18948707 Review.
-
The license to pair: identification of meiotic pairing sites in Drosophila.Chromosoma. 1996 Sep;105(3):135-41. doi: 10.1007/BF02509494. Chromosoma. 1996. PMID: 8781181 Review.
Cited by
-
A specific family of interspersed repeats (SINEs) facilitates meiotic synapsis in mammals.Mol Cytogenet. 2013 Jan 1;6(1):1. doi: 10.1186/1755-8166-6-1. Mol Cytogenet. 2013. PMID: 23276256 Free PMC article.
-
High-resolution transcriptional profiling of Anopheles gambiae spermatogenesis reveals mechanisms of sex chromosome regulation.Sci Rep. 2019 Oct 16;9(1):14841. doi: 10.1038/s41598-019-51181-1. Sci Rep. 2019. PMID: 31619757 Free PMC article.
-
Dynamic localization of DNA topoisomerase I and its functional relevance during Drosophila development.G3 (Bethesda). 2021 Sep 6;11(9):jkab202. doi: 10.1093/g3journal/jkab202. G3 (Bethesda). 2021. PMID: 34544118 Free PMC article.
-
Homologous chromosomes are stably conjoined for Drosophila male meiosis I by SUM, a multimerized protein assembly with modules for DNA-binding and for separase-mediated dissociation co-opted from cohesin.PLoS Genet. 2022 Dec 8;18(12):e1010547. doi: 10.1371/journal.pgen.1010547. eCollection 2022 Dec. PLoS Genet. 2022. PMID: 36480577 Free PMC article.
-
Separase Is Required for Homolog and Sister Disjunction during Drosophila melanogaster Male Meiosis, but Not for Biorientation of Sister Centromeres.PLoS Genet. 2016 Apr 27;12(4):e1005996. doi: 10.1371/journal.pgen.1005996. eCollection 2016 Apr. PLoS Genet. 2016. PMID: 27120695 Free PMC article.
References
-
- Appels, R., and A. J. Hilliker, 1982. The cytogenetic boundaries of the rDNA region within heterochromatin of the X chromosome of Drosophila melanogaster and their relation to male meiotic pairing sites. Genet. Res. 39: 149–156. - PubMed
-
- Ashburner, M., K. G. Golic and R. S. Hawley, 2005. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

