In vivo functional specificity and homeostasis of Drosophila 14-3-3 proteins
- PMID: 17660572
- PMCID: PMC2013677
- DOI: 10.1534/genetics.107.072280
In vivo functional specificity and homeostasis of Drosophila 14-3-3 proteins
Abstract
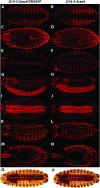
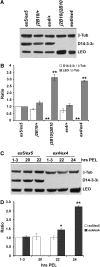
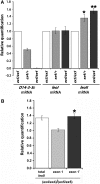
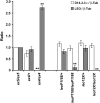
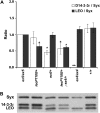
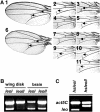
The functional specialization or redundancy of the ubiquitous 14-3-3 proteins constitutes a fundamental question in their biology and stems from their highly conserved structure and multiplicity of coexpressed isotypes. We address this question in vivo using mutations in the two Drosophila 14-3-3 genes, leonardo (14-3-3zeta) and D14-3-3epsilon. We demonstrate that D14-3-3epsilon is essential for embryonic hatching. Nevertheless, D14-3-3epsilon null homozygotes survive because they upregulate transcripts encoding the LEOII isoform at the time of hatching, compensating D14-3-3epsilon loss. This novel homeostatic response explains the reported functional redundancy of the Drosophila 14-3-3 isotypes and survival of D14-3-3epsilon mutants. The response appears unidirectional, as D14-3-3epsilon elevation upon LEO loss was not observed and elevation of leo transcripts was stage and tissue specific. In contrast, LEO levels are not changed in the wing disks, resulting in the aberrant wing veins characterizing D14-3-3epsilon mutants. Nevertheless, conditional overexpression of LEOI, but not of LEOII, in the wing disk can partially rescue the venation deficits. Thus, excess of a particular LEO isoform can functionally compensate for D14-3-3epsilon loss in a cellular-context-specific manner. These results demonstrate functional differences both among Drosophila 14-3-3 proteins and between the two LEO isoforms in vivo, which likely underlie differential dimer affinities toward 14-3-3 targets.
Figures

References
-
- Aitken, A., 1995. 14–3-3 proteins on the MAP. Trends Biochem. Sci. 20: 95–97. - PubMed
-
- Aitken, A., S. Howell, D. Jones, J. Madrazo and Y. Patel, 1995. 14–3-3 α and δ are the phosphorylated forms of Raf-activating 14–3-3 β and ζ. J. Biol. Chem. 270: 5706–5709. - PubMed
-
- Aitken, A., H. Baxter, T. Dubios, S. Clokie, S. Mackie et al., 2002. 14–3-3 proteins in cell regulation. Biochem. Soc. Trans. 30: 351–360. - PubMed
-
- Baxter, H. C., W.-G. Liu, J. L. Forster, A. Aitken and J. R. Fraser, 2002. Immunolocalisation of 14–3-3 isoforms in normal and scrapie-infected murine brain. Neuroscience 109: 5–14. - PubMed
-
- Benton, R., and D. St Johnston, 2003. Drosophila PAR-1 and 14–3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115: 691–704. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

