Tertiary structure and function of an RNA motif required for plant vascular entry to initiate systemic trafficking
- PMID: 17660743
- PMCID: PMC1952227
- DOI: 10.1038/sj.emboj.7601812
Tertiary structure and function of an RNA motif required for plant vascular entry to initiate systemic trafficking
Abstract
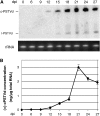
Vascular entry is a decisive step for the initiation of long-distance movement of infectious and endogenous RNAs, silencing signals and developmental/defense signals in plants. However, the mechanisms remain poorly understood. We used Potato spindle tuber viroid (PSTVd) as a model to investigate the direct role of the RNA itself in vascular entry. We report here the identification of an RNA motif that is required for PSTVd to traffic from nonvascular into the vascular tissue phloem to initiate systemic infection. This motif consists of nucleotides U/C that form a water-inserted cis Watson-Crick/Watson-Crick base pair flanked by short helices that comprise canonical Watson-Crick/Watson-Crick base pairs. This tertiary structural model was inferred by comparison with X-ray crystal structures of similar motifs in rRNAs and is supported by combined mutagenesis and covariation analyses. Hydration pattern analysis suggests that water insertion induces a widened minor groove conducive to protein and/or RNA interactions. Our model and approaches have broad implications to investigate the RNA structural motifs in other RNAs for vascular entry and to study the basic principles of RNA structure-function relationships.
Figures





Similar articles
-
A three-dimensional RNA motif in Potato spindle tuber viroid mediates trafficking from palisade mesophyll to spongy mesophyll in Nicotiana benthamiana.Plant Cell. 2011 Jan;23(1):258-72. doi: 10.1105/tpc.110.081414. Epub 2011 Jan 21. Plant Cell. 2011. PMID: 21258006 Free PMC article.
-
Tertiary structural and functional analyses of a viroid RNA motif by isostericity matrix and mutagenesis reveal its essential role in replication.J Virol. 2006 Sep;80(17):8566-81. doi: 10.1128/JVI.00837-06. J Virol. 2006. PMID: 16912306 Free PMC article.
-
Distinct RNA motifs mediate systemic RNA trafficking.Plant Signal Behav. 2008 Jan;3(1):58-9. doi: 10.4161/psb.3.1.4890. Plant Signal Behav. 2008. PMID: 19704772 Free PMC article.
-
An innate twist between Crick's wobble and Watson-Crick base pairs.RNA. 2013 Aug;19(8):1038-53. doi: 10.1261/rna.036905.112. RNA. 2013. PMID: 23861536 Free PMC article. Review.
-
Viroid trafficking: a small RNA makes a big move.Curr Opin Plant Biol. 2005 Dec;8(6):606-12. doi: 10.1016/j.pbi.2005.09.001. Epub 2005 Sep 21. Curr Opin Plant Biol. 2005. PMID: 16181802 Review.
Cited by
-
Viroid intercellular trafficking: RNA motifs, cellular factors and broad impacts.Viruses. 2009 Sep;1(2):210-21. doi: 10.3390/v1020210. Epub 2009 Sep 1. Viruses. 2009. PMID: 21994546 Free PMC article.
-
A three-dimensional RNA motif in Potato spindle tuber viroid mediates trafficking from palisade mesophyll to spongy mesophyll in Nicotiana benthamiana.Plant Cell. 2011 Jan;23(1):258-72. doi: 10.1105/tpc.110.081414. Epub 2011 Jan 21. Plant Cell. 2011. PMID: 21258006 Free PMC article.
-
Allelic RNA Motifs in Regulating Systemic Trafficking of Potato Spindle Tuber Viroid.Viruses. 2018 Mar 30;10(4):160. doi: 10.3390/v10040160. Viruses. 2018. PMID: 29601476 Free PMC article.
-
Mobility of Transgenic Nucleic Acids and Proteins within Grafted Rootstocks for Agricultural Improvement.Front Plant Sci. 2012 Mar 2;3:39. doi: 10.3389/fpls.2012.00039. eCollection 2012. Front Plant Sci. 2012. PMID: 22645583 Free PMC article.
-
Non-coding RNAs in Intercellular and Systemic Signaling.Front Plant Sci. 2012 Jul 5;3:141. doi: 10.3389/fpls.2012.00141. eCollection 2012. Front Plant Sci. 2012. PMID: 22783264 Free PMC article. No abstract available.
References
-
- Auffinger P, Hashem Y (2007) SwS a solvation web service for nucleic acids. Bioinformatics 17: 325–333 - PubMed
-
- Batey RT, Sagar MB, Doudna JA (2001) Structural and energetic analysis of RNA recognition by a universally conserved protein from the signal recognition particle. J Mol Biol 307: 229–246 - PubMed
-
- Branch AD, Robertson HD (1984) A replication cycle for viroids and other small infectious RNAs. Science 223: 450–455 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

