Tachyphylaxis of the ECL-cell response to PACAP: receptor desensitization and/or depletion of secretory products
- PMID: 17660849
- PMCID: PMC1978265
- DOI: 10.1038/sj.bjp.0707385
Tachyphylaxis of the ECL-cell response to PACAP: receptor desensitization and/or depletion of secretory products
Abstract
Background and purpose: Rat stomach ECL cells secrete histamine and pancreastatin in response to gastrin and pituitary adenylate cyclase-activating peptide-27 (PACAP). This study applies microdialysis to explore how ECL cells in situ respond to PACAP and gastrin.
Experimental approach: Both peptides were administered by microinfusion into the gastric submucosa. The microdialysate was analysed for histamine and pancreastatin (ECL-cell markers) and for somatostatin (D-cell marker).
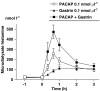
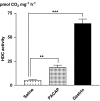
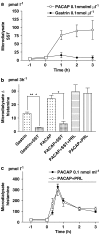
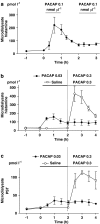
Key results: Microinfusion of PACAP (0.01-0.3 nmol microl(-1)) raised microdialysate histamine and pancreastatin dose-dependently. The response was powerful but short-lived. The response to gastrin was sustained at all doses tested. It is unlikely that the transient nature of the histamine response to PACAP reflects inadequate histamine synthesis, since the pancreastatin response to PACAP was short-lived too, and both gastrin and PACAP activated ECL-cell histidine decarboxylase. Unlike gastrin, PACAP mobilized somatostatin. Co-infusion of somatostatin abolished the histamine-mobilizing effect of PACAP. However, pretreatment with the somatostatin receptor type-2 antagonist (PRL-2903) did not prolong the histamine response to PACAP, suggesting that mobilization of somatostatin does not explain the transient nature of the response. Repeated administration of 0.1 nmol microl(-1) of PACAP (1 h infusions, 1 h intervals) failed to induce a second histamine response. Pretreatment with a low dose of PACAP (0.03 nmol microl(-1)) abolished the response to a subsequent near-maximal PACAP challenge (0.3 nmol microl(-1)).
Conclusion: The transient nature of the histamine response to PACAP reflects desensitization of the PACAP receptor and/or exhaustion of a specific storage compartment that responds to PACAP but not to gastrin.
Figures




Similar articles
-
Somatostatin, misoprostol and galanin inhibit gastrin- and PACAP-stimulated secretion of histamine and pancreastatin from ECL cells by blocking specific Ca2+ channels.Regul Pept. 2005 Aug 15;130(1-2):81-90. doi: 10.1016/j.regpep.2005.04.002. Regul Pept. 2005. PMID: 15935492
-
Gastric submucosal microdialysis: a method to study gastrin- and food-evoked mobilization of ECL-cell histamine in conscious rats.Regul Pept. 2000 Jan 29;86(1-3):113-23. doi: 10.1016/s0167-0115(99)00096-8. Regul Pept. 2000. PMID: 10672910
-
Neurohormonal regulation of histamine and pancreastatin secretion from isolated rat stomach ECL cells.Regul Pept. 1997 Aug 15;71(2):73-86. doi: 10.1016/s0167-0115(97)01018-5. Regul Pept. 1997. PMID: 9416989
-
Physiology of the ECL cells.Yale J Biol Med. 1998 May-Aug;71(3-4):163-71. Yale J Biol Med. 1998. PMID: 10461349 Free PMC article. Review.
-
CCK2 receptor antagonists: pharmacological tools to study the gastrin-ECL cell-parietal cell axis.Regul Pept. 1999 Mar 17;80(1-2):1-12. doi: 10.1016/s0167-0115(99)00008-7. Regul Pept. 1999. PMID: 10235629 Review.
Cited by
-
The development of multiple probe microdialysis sampling in the stomach.J Pharm Biomed Anal. 2008 Sep 10;48(1):20-6. doi: 10.1016/j.jpba.2008.04.019. Epub 2008 Apr 26. J Pharm Biomed Anal. 2008. PMID: 18539423 Free PMC article.
-
Presence and Effects of Pituitary Adenylate Cyclase Activating Polypeptide Under Physiological and Pathological Conditions in the Stomach.Front Endocrinol (Lausanne). 2018 Mar 19;9:90. doi: 10.3389/fendo.2018.00090. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29615974 Free PMC article. Review.
References
-
- Andersson K, Chen D, Håkanson R, Mattsson H, Sundler F. Enterochromaffin-like cells in the rat stomach: effect of α-FMH-evoked histamine depletion. A chemical, histochemical and electron microscopic study. Cell Tissue Res. 1992;270:7–13. - PubMed
-
- Björkqvist M, Bernsand M, Eliasson L, Håkanson R, Lindström E. Somatostatin, misoprostol and galanin inhibit gastrin- and PACAP-stimulated secretion of histamine and pancreastatin from ECL cells by blocking specific Ca2+ channels. Regul Pept. 2005;130:81–90. - PubMed
-
- Black JW, Shankley NP. How does gastrin act to stimulate oxyntic cell secretion. Trends Pharmacol Sci. 1987;8:486–490.
-
- Chen D, Monstein H-J, Nylander A-G, Zhao C-M, Sundler F, Håkanson R. Acute response of rat stomach enterochromaffin-like cells to gastrin. Secretory activation and adaptation. Gastroenterology. 1994;107:18–27. - PubMed
-
- Ekblad E, Ekelund M, Graffner H, Håkanson R, Sundler F. Peptide-containing nerve fibers in the stomach wall of rat and mouse. Gastroenterology. 1985;89:73–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

