Differences in extracellular matrix production and basic fibroblast growth factor response in skin fibroblasts from sporadic and familial Alzheimer's disease
- PMID: 17660861
- PMCID: PMC1933258
- DOI: 10.2119/2007-00034.Bellucci
Differences in extracellular matrix production and basic fibroblast growth factor response in skin fibroblasts from sporadic and familial Alzheimer's disease
Abstract
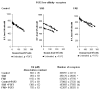
Extracellular matrix (ECM) molecules and growth factors, such as fibroblast growth factor (FGF), play a crucial role in Alzheimer's disease (AD). The purpose of this investigation was to determine whether phenotypic alterations in ECM production are present in non-neuronal AD cells associated with different FGF expression and response. Synthesis of glycosaminoglycans (GAG) and collagen were measured in skin fibroblasts from patients with familial, sporadic AD (FAD and SAD respectively), and from age-matched controls by radiolabeled precursors. Proteoglycans (PG), metalloprotease (MMP)-1, and FGF gene expressions were measured by reverse transcription-polymerase chain reaction. The results showed different ECM neosynthesis and mRNA levels in the two AD fibroblast populations. FAD accumulated more collagen and secreted less GAG than SAD. Biglycan PG was upregulated in FAD while betaglycan, syndecan, and decorin were markedly downregulated in SAD fibroblasts. We found a significant decrease of MMP1, more marked in FAD than in SAD fibroblasts. Constitutive FGF expression was greatly reduced in both pathological conditions (SAD>FAD). Moreover, an inverse high affinity/low affinity FGF receptor ratio between SAD and FAD fibroblasts was observed. FGF treatment differently modulated ECM molecule production and gene expression in the two cell populations. These observations in association with the changes in FGF gene expression and in the FGF receptor number, suggest that cellular mechanisms downstream from FGF receptor binding are involved in the two different forms of AD.
Figures
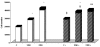
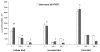
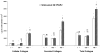

References
-
- Smith MA. Alzheimer disease. Int Rev Neurobiol. 1998;42:1–54. - PubMed
-
- Hardy J. Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci. 1997;20:154–9. - PubMed
-
- Castillo GM, Ngo C, Cummings J, Wight TN, Snow AD. Perlecan binds to the β-amyloid proteins (Aβ) of Alzheimer’s disease, accelerates Aβ fibril formation, and maintains Aβ fibril stability. J Neurochem. 1997;69:2452–65. - PubMed
-
- Bruckner G, Hausen D, Harting W, Drlicek M, Arendt T, Brauer K. Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer’s disease. Neuroscience. 1999;92:791–805. - PubMed
-
- Gerst JL, et al. Altered cell-matrix associated ADAM proteins in Alzheimer disease. J Neurosc Res. 2000;59:680–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
