From old organisms to new molecules: integrative biology and therapeutic targets in accelerated human ageing
- PMID: 17660942
- PMCID: PMC2773833
- DOI: 10.1007/s00018-007-7123-x
From old organisms to new molecules: integrative biology and therapeutic targets in accelerated human ageing
Abstract
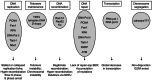
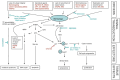
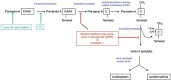
Understanding the basic biology of human ageing is a key milestone in attempting to ameliorate the deleterious consequences of old age. This is an urgent research priority given the global demographic shift towards an ageing population. Although some molecular pathways that have been proposed to contribute to ageing have been discovered using classical biochemistry and genetics, the complex, polygenic and stochastic nature of ageing is such that the process as a whole is not immediately amenable to biochemical analysis. Thus, attempts have been made to elucidate the causes of monogenic progeroid disorders that recapitulate some, if not all, features of normal ageing in the hope that this may contribute to our understanding of normal human ageing. Two canonical progeroid disorders are Werner's syndrome and Hutchinson-Gilford progeroid syndrome (also known as progeria). Because such disorders are essentially phenocopies of ageing, rather than ageing itself, advances made in understanding their pathogenesis must always be contextualised within theories proposed to help explain how the normal process operates. One such possible ageing mechanism is described by the cell senescence hypothesis of ageing. Here, we discuss this hypothesis and demonstrate that it provides a plausible explanation for many of the ageing phenotypes seen in Werner's syndrome and Hutchinson-Gilford progeriod syndrome. The recent exciting advances made in potential therapies for these two syndromes are also reviewed.
Figures
Similar articles
-
Prematurely aged children: molecular alterations leading to Hutchinson-Gilford progeria and Werner syndromes.Curr Aging Sci. 2008 Dec;1(3):202-12. doi: 10.2174/1874609810801030202. Curr Aging Sci. 2008. PMID: 20021393 Review.
-
Model of human aging: recent findings on Werner's and Hutchinson-Gilford progeria syndromes.Clin Interv Aging. 2008;3(3):431-44. doi: 10.2147/cia.s1957. Clin Interv Aging. 2008. PMID: 18982914 Free PMC article. Review.
-
Werner and Hutchinson-Gilford progeria syndromes: mechanistic basis of human progeroid diseases.Nat Rev Mol Cell Biol. 2007 May;8(5):394-404. doi: 10.1038/nrm2161. Nat Rev Mol Cell Biol. 2007. PMID: 17450177 Review.
-
Human progeroid syndromes, aging and cancer: new genetic and epigenetic insights into old questions.Cell Mol Life Sci. 2007 Jan;64(2):155-70. doi: 10.1007/s00018-006-6349-3. Cell Mol Life Sci. 2007. PMID: 17131053 Free PMC article. Review.
-
Genetic alterations in accelerated ageing syndromes. Do they play a role in natural ageing?Int J Biochem Cell Biol. 2005 May;37(5):947-60. doi: 10.1016/j.biocel.2004.10.011. Epub 2004 Dec 15. Int J Biochem Cell Biol. 2005. PMID: 15743670 Review.
Cited by
-
The Drosophila orthologue of progeroid human WRN exonuclease, DmWRNexo, cleaves replication substrates but is inhibited by uracil or abasic sites : analysis of DmWRNexo activity in vitro.Age (Dordr). 2013 Jun;35(3):793-806. doi: 10.1007/s11357-012-9411-0. Epub 2012 May 5. Age (Dordr). 2013. PMID: 22562358 Free PMC article.
-
Accelerated ageing: from mechanism to therapy through animal models.Transgenic Res. 2009 Feb;18(1):7-15. doi: 10.1007/s11248-008-9226-z. Epub 2008 Nov 18. Transgenic Res. 2009. PMID: 19015945 Review.
-
Clock genes, hair growth and aging.Aging (Albany NY). 2010 Mar 31;2(3):122-8. doi: 10.18632/aging.100130. Aging (Albany NY). 2010. PMID: 20375466 Free PMC article. Review.
-
Premature aging.Cell Mol Life Sci. 2009 Sep;66(18):3091-4. doi: 10.1007/s00018-009-0091-6. Epub 2009 Jul 18. Cell Mol Life Sci. 2009. PMID: 19618112 Free PMC article. Review. No abstract available.
-
Hutchinson-Gilford Progeria Syndrome: A Premature Aging Disease.Mol Neurobiol. 2018 May;55(5):4417-4427. doi: 10.1007/s12035-017-0610-7. Epub 2017 Jun 28. Mol Neurobiol. 2018. PMID: 28660486 Review.
References
-
- Mills H. In: Fit for the future: the prevention of dependency in later life. Prophet H., editor. London: Report of the Continuing Care Conference; 1998.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

