Impaired clearance of methotrexate in organic anion transporter 3 (Slc22a8) knockout mice: a gender specific impact of reduced folates
- PMID: 17660957
- PMCID: PMC2820254
- DOI: 10.1007/s11095-007-9407-0
Impaired clearance of methotrexate in organic anion transporter 3 (Slc22a8) knockout mice: a gender specific impact of reduced folates
Abstract
Purpose: To elucidate the role of the renal basolateral transporter, Oat3, in the disposition of methotrexate.
Materials and methods: Chinese hamster ovary cells expressing mouse Oat3 were used to determine kinetics and specificity of inhibition of methotrexate transport. Methotrexate clearance was then examined in vivo in wildtype and Oat3 knockout mice.
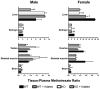
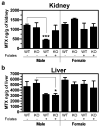
Results: NSAIDs, beta-lactams, and uremic toxins inhibited mOat3-mediated methotrexate uptake by 70-100%, while folate, leucovorin, and 5-methyltetrahydrofolate inhibited transport by 25-50%. A Km of 60.6 +/- 9.3 microM for methotrexate transport was determined. Oat3 knockout mice exhibited reduced methotrexate-to-inulin clearance ratios versus wildtype. Male wildtype mice, but not knockouts or females, demonstrated significantly accelerated methotrexate clearance in response to reduced folates. Reduced folates also markedly inhibited hepatic methotrexate accumulation in males, but not females, and the response was independent of Oat3 function.
Conclusions: Oat3 contributes to methotrexate clearance, but represents only one component responsible for methotrexate's elimination. Therefore, in patients, dysfunctional hOAT3 polymorphisms or drug competition for hOAT3 transport may severely impact methotrexate elimination only when redundant means of methotrexate removal are also compromised. Furthermore, the present findings suggest that reduced-folate administration only influences methotrexate disposition in males, with the renal reduced-folate response influenced by OAT3 function.
Figures
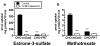




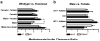
Similar articles
-
Organic anion transporter 3 (Oat3/Slc22a8) knockout mice exhibit altered clearance and distribution of penicillin G.Am J Physiol Renal Physiol. 2007 Oct;293(4):F1332-41. doi: 10.1152/ajprenal.00319.2007. Epub 2007 Aug 8. Am J Physiol Renal Physiol. 2007. PMID: 17686950 Free PMC article.
-
Organic anion transporter 3 (oat3/slc22a8) interacts with carboxyfluoroquinolones, and deletion increases systemic exposure to ciprofloxacin.Mol Pharmacol. 2008 Jul;74(1):122-31. doi: 10.1124/mol.107.042853. Epub 2008 Apr 1. Mol Pharmacol. 2008. PMID: 18381565 Free PMC article.
-
Inhibition of Methotrexate Uptake via Organic Anion Transporters OAT1 and OAT3 by Glucuronides of Nonsteroidal Anti-inflammatory Drugs.Biol Pharm Bull. 2017;40(6):926-931. doi: 10.1248/bpb.b16-00970. Biol Pharm Bull. 2017. PMID: 28566636
-
Renal conservation of folates role of folate transport proteins.Vitam Horm. 2008;79:185-202. doi: 10.1016/S0083-6729(08)00406-8. Vitam Horm. 2008. PMID: 18804695 Review.
-
Identification and Quantitative Assessment of Uremic Solutes as Inhibitors of Renal Organic Anion Transporters, OAT1 and OAT3.Mol Pharm. 2016 Sep 6;13(9):3130-40. doi: 10.1021/acs.molpharmaceut.6b00332. Epub 2016 Aug 9. Mol Pharm. 2016. PMID: 27467266 Review.
Cited by
-
Concomitant Use of High-dose Methotrexate and Glycyrrhizin Affects Pharmacokinetics of Methotrexate, Resulting in Hepatic Toxicity.In Vivo. 2021 Jul-Aug;35(4):2163-2169. doi: 10.21873/invivo.12487. In Vivo. 2021. PMID: 34182493 Free PMC article.
-
Genetic Heterogeneity of SLC22 Family of Transporters in Drug Disposition.J Pers Med. 2018 Apr 16;8(2):14. doi: 10.3390/jpm8020014. J Pers Med. 2018. PMID: 29659532 Free PMC article. Review.
-
Reduced renal clearance of cefotaxime in asians with a low-frequency polymorphism of OAT3 (SLC22A8).J Pharm Sci. 2013 Sep;102(9):3451-7. doi: 10.1002/jps.23581. Epub 2013 May 6. J Pharm Sci. 2013. PMID: 23649425 Free PMC article.
-
Choroid Plexus and Drug Removal Mechanisms.AAPS J. 2021 May 3;23(3):61. doi: 10.1208/s12248-021-00587-9. AAPS J. 2021. PMID: 33942198 Review.
-
Active Hydrophilic Components of the Medicinal Herb Salvia miltiorrhiza (Danshen) Potently Inhibit Organic Anion Transporters 1 (Slc22a6) and 3 (Slc22a8).Evid Based Complement Alternat Med. 2012;2012:872458. doi: 10.1155/2012/872458. Epub 2012 Jul 15. Evid Based Complement Alternat Med. 2012. PMID: 22844339 Free PMC article.
References
-
- Methotrexate DRUGDEX® Evaluations. n.d. Thomson Micro-medex. Mar. 14, 2007. Available at: http://www.thomsonhc.com.
-
- Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. - PubMed
-
- White JC, Goldman ID. Mechanism of action of methotrexate. IV. Free intracellular methotrexate required to suppress dihydrofolate reduction to tetrahydrofolate by Ehrlich ascites tumor cells in vitro. Mol. Pharmacol. 1976;12:711–719. - PubMed
-
- White JC, Loftfield S, Goldman ID. The mechanism of action of methotrexate. III. Requirement of free intracellular methotrexate for maximal suppression of (14C)formate incorporation into nucleic acids and protein. Mol. Pharmacol. 1975;11:287–297. - PubMed
-
- Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol. Rev. 2004;84:987–1049. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

