The in- or exclusion of non-breast cancer related death and contralateral breast cancer significantly affects estimated outcome probability in early breast cancer
- PMID: 17661169
- PMCID: PMC2668629
- DOI: 10.1007/s10549-007-9681-x
The in- or exclusion of non-breast cancer related death and contralateral breast cancer significantly affects estimated outcome probability in early breast cancer
Abstract
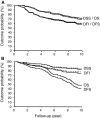
A wide variation of definitions of recurrent disease and survival are used in the analyses of outcome of patients with early breast cancer. Explicit definitions with details both on endpoints and censoring are provided in less than half of published studies. We evaluated the effects of various definitions of survival and recurrent disease on estimated outcome in a prospectively determined cohort of 463 patients with primary breast cancer. Outcome estimates were determined both by the Kaplan-Meier and a competing risk method. In- or exclusion of contralateral breast cancer or non-disease related death in the definition of recurrent disease or survival significantly affects estimated outcome probability. The magnitude of this finding was dependent on patient-, tumour-, and treatment characteristics. Knowledge of the contribution of non-disease related death or contralateral breast cancer to estimated recurrent disease rate and overall death rate is indispensable for a correct interpretation and comparison of outcome analyses.
Figures
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PMC', 'value': 'PMC2033978', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC2033978/'}, {'type': 'PubMed', 'value': '7640241', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/7640241/'}]}
- Altman DG, De Stavola BL, Love SB, Stepniewska KA (1995) Review of survival analyses published in cancer journals. Br J Cancer 72:511–518 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0140-6736(98)03301-7', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0140-6736(98)03301-7'}, {'type': 'PubMed', 'value': '9752815', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9752815/'}]}
- Altman DG, De Stavola BL, Love SB, Stepniewska KA (1998) Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 352:930–942 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '2644532', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/2644532/'}]}
- Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, Dimitrov NV, Wolmark N, Wickerham DL, Fisher ER, Wolmark N (1989) A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med 320:479–484 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1093/jnci/89.22.1673', 'is_inner': False, 'url': 'https://doi.org/10.1093/jnci/89.22.1673'}, {'type': 'PubMed', 'value': '9390536', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9390536/'}]}
- Fisher B, Dignam J, Wolmark N, DeCillis A, Emir B, Wickerham DL, Bryant J, Dimitrov NV, Abramson N, Atkins JN, Shibata H, Deschenes L, Margolese RG (1997) Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst 89:1673–1682 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0140-6736(04)16981-X', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0140-6736(04)16981-x'}, {'type': 'PubMed', 'value': '15351193', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15351193/'}]}
- Fisher B, Jeong JH, Bryant J, Anderson S, Dignam J, Fisher ER, Wolmark N (2004) Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from national surgical adjuvant breast and bowel project randomised clinical trials. Lancet 364:858–868 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

