IFN-gamma-induced IDO and WRS expression in microglia is differentially regulated by IL-4
- PMID: 17661345
- PMCID: PMC2486430
- DOI: 10.1002/glia.20544
IFN-gamma-induced IDO and WRS expression in microglia is differentially regulated by IL-4
Abstract
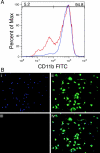
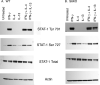
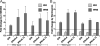
Indoleamine 2,3-dioxygenase (IDO), a tryptophan catabolizing enzyme, has been implicated in the pathogenesis of various neurological disorders. IDO expression is induced by IFN-gamma and leads to neurotoxicity by generating quinolinic acid. Additionally, it inhibits the immune response through both tryptophan depletion and generating other tryptophan catabolites. IL-4 and IL-13 have been shown to control IDO expression by antagonizing the effects of IFN-gamma in different cell types. Here, we investigated the effects of these cytokines on IDO expression in microglia. Interestingly, we observed that both IL-4 and IL-13 greatly enhanced IFN-gamma-induced IDO expression. However, tryptophanyl-tRNA synthetase (WRS), which is coinduced with IDO by IFN-gamma, is downregulated by IL-4 and IL-13. The effect of IL-4 and IL-13 was independent of STAT-6. Modulation of IDO but not WRS was eliminated by inhibition of protein phosphatase 2A (PP2A) activity. The phosphatidylinositol 3-kinase (PI3K) pathway further differentiated the regulation of these two enzymes, as inhibiting the PI3K pathway eliminated IFN-gamma induction of IDO, whereas such inhibition greatly enhanced WRS expression. These findings show discordance between modulations of expression of two distinct enzymes utilizing tryptophan as a common substrate, and raise the possibility of their involvement in regulating immune responses in various neurological disorders.
Copyright (c) 2007 Wiley-Liss, Inc.
Figures
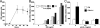





References
-
- Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–91. - PubMed
-
- Bara H, Hainfellner JA, Kepplinger B, Mazal PR, Schmid H, Budka H. Kynurenic acid metabolism in the brain of HIV-1 infected patients. J Neural Transm. 2000;107(10):1127–38. - PubMed
-
- Boasso A, Herbeuval JP, Hardy AW, Winkler C, Shearer GM. Regulation of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells. Blood. 2005;105(4):1574–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

