Investigation of the C-terminal redox center of high-Mr thioredoxin reductase by protein engineering and semisynthesis
- PMID: 17661444
- PMCID: PMC3682222
- DOI: 10.1021/bi7004812
Investigation of the C-terminal redox center of high-Mr thioredoxin reductase by protein engineering and semisynthesis
Abstract
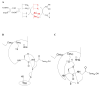
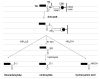
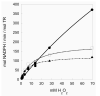
High-molecular weight thioredoxin reductases (TRs) catalyze the reduction of the redox-active disulfide bond of thioredoxin, but an important difference in the TR family is the sequence of the C-terminal redox-active tetrapeptide that interacts directly with thioredoxin, especially the presence or absence of a selenocysteine (Sec) residue in this tetrapeptide. In this study, we have employed protein engineering techniques to investigate the C-terminal redox-active tetrapeptides of three different TRs: mouse mitochondrial TR (mTR3), Drosophila melanogaster TR (DmTR), and the mitochondrial TR from Caenorhabditis elegans (CeTR2), which have C-terminal tetrapeptide sequences of Gly-Cys-Sec-Gly, Ser-Cys-Cys-Ser, and Gly-Cys-Cys-Gly, respectively. Three different types of mutations and chemical modifications were performed in this study: insertion of alanine residues between the cysteine residues of the Cys-Cys or Cys-Sec dyads, modification of the charge at the C-terminus, and altering the position of the Sec residue in the mammalian enzyme. The results show that mTR3 is quite accommodating to insertion of alanine residues into the Cys-Sec dyad, with only a 4-6-fold drop in catalytic activity. In contrast, the activity of both DmTR and CeTR2 was reduced 100-300-fold when alanine residues were inserted into the Cys-Cys dyad. We have tested the importance of a salt bridge between the C-terminus and a basic residue that was proposed for orienting the Cys-Sec dyad of mTR3 for proper catalytic position by changing the C-terminal carboxylate to a carboxamide. The result is an enzyme with twice the activity as the wild-type mammalian enzyme. A similar result was achieved when the C-terminal carboxylate of DmTR was converted to a hydroxamic acid or a thiocarboxylate. Last, reversing the positions of the Cys and Sec residues in the catalytic dyad resulted in a 100-fold loss of catalytic activity. Taken together, the results support our previous model of Sec as the leaving group during reduction of the C-terminus during the catalytic cycle.
Figures

References
-
- Arscott LD, Gromer S, Schirmer RH, Becker K, Williams CH., Jr. The mechanism of thioredoxin reductase from human placenta is similar to the mechanisms of lipoamide dehydrogenase and glutathione reductase and is distinct from the mechanism of thioredoxin reductase from Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3621–3626. - PMC - PubMed
-
- Bauer H, Massey V, Arscott LD, Schirmer RH, Ballou DP, Williams CH., Jr. The mechanism of high Mr thioredoxin reductase from Drosophila melanogaster. J. Biol. Chem. 2003;278:33020–33028. - PubMed
-
- Wang PF, Arscott LD, Gilberger TW, Muller S, Williams CH., Jr. Thioredoxin reductase from Plasmodium falciparum: evidence for interaction between the C-terminal cysteine residues and the active site disulfide-dithiol. Biochemistry. 1999;38:3187–3196. - PubMed
-
- Hudaky I, Gaspari Z, Carugo O, Cemazar M, Pongor S, Perczel A. Vicinal disulfide bridge conformers by experimental methods and by ab initio and DFT molecular computations. Proteins. 2004;55:152–168. - PubMed
-
- Carugo O, Cemazar M, Zahariev S, Hudaky I, Gaspari Z, Perczel A, Pongor S. Vicinal disulfide turns. Protein Eng. 2003;16:637–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

