Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11
- PMID: 17662044
- PMCID: PMC2265989
- DOI: 10.1111/j.1365-2567.2007.02647.x
Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11
Abstract
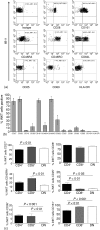
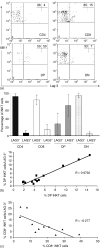
Identification of human CD1d-restricted T-cell receptor (TCR)-invariant natural killer T (iNKT) cells has been dependent on utilizing combinations of monoclonal antibodies or CD1d tetramers, which do not allow for the most specific analysis of this T-cell subpopulation. A novel monoclonal antibody (clone 6B11), specific for the invariant CDR3 loop of human canonical Valpha24Jalpha18 TCR alpha chain, was developed and used to specifically characterize iNKT cells. In healthy individuals studied for up to 1 year, a wide but stable frequency of circulating iNKT cells (range: 0.01-0.92%) was observed, with no differences in frequency by gender. Four stable iNKT cell subsets were characterized in peripheral blood based on the expression of CD4 and CD8, with CD8(+) iNKT cells being a phenotypic and functionally different subset from CD4(+) and double negative iNKT cells; in particular, LAG-3 was preferentially expressed on CD8(+) iNKT cells. In addition, a strong negative linear correlation between the frequency of total iNKT cells and percentage of the CD4(+) subset was observed. In terms of their potential association with disease, patients at risk for type 1 diabetes had significantly expanded frequencies of double negative iNKT cells when compared to matched controls and first-degree relatives. Moreover, peripheral blood CD4(+) iNKT cells were the highest producers of interleukin-4, while the production of interferon-gamma and tumour necrosis factor-alpha was similar amongst all iNKT cell subsets. These differences in iNKT cell subsets suggest that in humans the relative ratio of iNKT cell subsets may influence susceptibility vs. resistance to immune-mediated diseases.
Figures


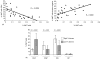

References
-
- Wilson SB, Delovitch TL. Janus-like role of regulatory iNKT cells in autoimmune disease and tumor immunity. Nat Rev Immunol. 2003;3:211–22. - PubMed
-
- Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21:573–83. - PubMed
-
- Chen H, Paul WE. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-γ upon activation by anti-CD3 or CD1. J Immunol. 1997;159:2240–9. - PubMed
-
- Porcelli SA, Modlin RL. The CD1 system. antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. - PubMed
-
- Bendelac A, Rivera MN, Park HS, Roark JH. Mouse CD1-specific NK1 T cells: development, specifity and function. Annu Rev Immunol. 1997;15:535–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

