Estrogen receptor beta in the brain: from form to function
- PMID: 17662459
- PMCID: PMC2374745
- DOI: 10.1016/j.brainresrev.2007.05.013
Estrogen receptor beta in the brain: from form to function
Abstract
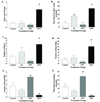
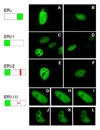
Estrogens have numerous effects on the brain, both in adulthood and during development. These actions of estrogen are mediated by two distinct estrogen receptor (ER) systems, ER alpha (ERalpha) and ER beta (ERbeta). In brain, ERalpha plays a critical role in regulating reproductive neuroendocrine function and behavior, however, a definitive role for ERbeta in any neurobiological function has been slow in forthcoming. Clues to the function of ERbeta in the central nervous system can be gleaned from the neuroanatomical distribution of ERbeta and the phenotypes of neurons that express ERbeta. ERbeta immunoreactivity has been found in populations of GnRH, CRH, vasopressin, oxytocin and prolactin containing neurons in the hypothalamus. Utilizing subtype-selective estrogen receptor agonists can help determine the roles for ERbeta in non-reproductive behaviors in rat models. ERbeta-selective agonists exert potent anxiolytic activity when animals were tested in a number of behavioral paradigms. Consistent with this, ERbeta-selective agonists also inhibited the ACTH and corticosterone response to stress. In contrast, ERalpha selective agonists were found to be anxiogenic and correspondingly increased the hormonal stress response. Taken together, our studies implicate ERbeta as an important modulator of some non-reproductive neurobiological systems. The molecular and neuroanatomical targets of estrogen that are mediated by ERbeta remain to be determined. A number of splice variants of ERbeta mRNA have been reported in brain tissue. Imaging of eGFP labeled chimeric receptor proteins transfected into cell lines shows that ERbeta splice variation can alter trafficking patterns and function. The originally described ERbeta (herein termed ERbeta1) is characterized by possessing a high affinity for estradiol. Similar to ERalpha, it is localized in the nucleus and is trafficked to nuclear sites termed "hyperspeckles" following ligand binding. In contrast, ERbeta2 contains an 18 amino acid insert within the ligand-binding domain and as a result can be best described as a low affinity form of ERbeta. A delta3 (delta3) variant of ERbeta has a deletion of the 3rd exon (coding for the second half of the DNA-binding domain) and as a result does not bind an estrogen response element in DNA. delta3 variants are trafficked to a unique low abundance and larger nuclear site following ligand binding. A delta4 (delta4) variant lacks exon 4 and as a result is localized to the cytoplasm. The amount of individual splice variant mRNAs varies depending upon brain region. Examination of neuropeptide promoter regulation by ERbeta splice variants demonstrates that ERbeta functions as a constitutively active transcription factor. Moreover, it appears that splice variation of ERbeta alters its ability to regulate transcription in a promoter-dependent and ligand-dependent fashion.
Figures

References
-
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol. 2004;16:319–324. - PubMed
-
- Angold A, Worthman CW. Puberty onset of gender differences in rates of depression: a developmental, epidemiologic and neuroendocrine perspective. J Affect Disord. 1993;29:145–158. - PubMed
-
- Arteaga-Lopez PR, Dominguez R, Cerbon MA, Mendoza-Rodriguez CA, Cruz ME. Differential mRNA expression of alpha and beta estrogen receptor isoforms and GnRH in the left and right side of the preoptic and anterior hypothalamic area during the estrous cycle of the rat. Endocrine. 2003;21:251–260. - PubMed
-
- Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999;26:260–267. - PubMed
-
- Bingaman EW, Magnuson DJ, Gray TS, Handa RJ. Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinology. 1994;59:228–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

