Tetrapeptide inhibitors of the glutamate vesicular transporter (VGLUT)
- PMID: 17662605
- PMCID: PMC2001293
- DOI: 10.1016/j.bmcl.2007.07.006
Tetrapeptide inhibitors of the glutamate vesicular transporter (VGLUT)
Abstract
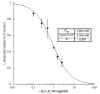
Quinoline-2,4-dicarboxylic acids (QDCs) bearing lipophilic substituents in the 6- or 7-position were shown to be inhibitors of the glutamate vesicular transporter (VGLUT). Using the arrangement of the QDC lipophilic substituents as a template, libraries of X(1)X(2)EF and X(1)X(2)EW tetrapeptides were synthesized and tested as VGLUT inhibitors. The peptides QIEW and WNEF were found to be the most potent. Further stereochemical deconvolution of these two peptides showed dQlIdElW to be the best inhibitor (K(i)=828+/-252 microM). Modeling and overlay of the tetrapeptide inhibitors with the existing pharmacophore showed that H-bonding and lipophilic residues are important for VGLUT binding.
Figures





Similar articles
-
The development of benzo- and naphtho-fused quinoline-2,4-dicarboxylic acids as vesicular glutamate transporter (VGLUT) inhibitors reveals a possible role for neuroactive steroids.Bioorg Med Chem Lett. 2014 Feb 1;24(3):850-4. doi: 10.1016/j.bmcl.2013.12.086. Epub 2013 Dec 25. Bioorg Med Chem Lett. 2014. PMID: 24424130 Free PMC article.
-
Synthesis and in vitro pharmacology of substituted quinoline-2,4-dicarboxylic acids as inhibitors of vesicular glutamate transport.J Med Chem. 2002 May 23;45(11):2260-76. doi: 10.1021/jm010261z. J Med Chem. 2002. PMID: 12014964
-
Inhibitor of the glutamate vesicular transporter (VGLUT).Curr Med Chem. 2005;12(18):2041-56. doi: 10.2174/0929867054637635. Curr Med Chem. 2005. PMID: 16101493 Review.
-
Time of day-dependent sorting of the vesicular glutamate transporter to the plasma membrane.J Biol Chem. 2009 Feb 13;284(7):4300-7. doi: 10.1074/jbc.M805480200. Epub 2008 Dec 22. J Biol Chem. 2009. PMID: 19103593
-
The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids.Pflugers Arch. 2004 Feb;447(5):756-9. doi: 10.1007/s00424-003-1091-2. Epub 2003 May 16. Pflugers Arch. 2004. PMID: 12750892 Review.
Cited by
-
Expression of vesicular glutamate transporters type 1 and 2 in sensory and autonomic neurons innervating the mouse colorectum.J Comp Neurol. 2011 Nov 1;519(16):3346-66. doi: 10.1002/cne.22730. J Comp Neurol. 2011. PMID: 21800314 Free PMC article.
-
Vesicular Glutamate Transporter Inhibitors: Structurally Modified Brilliant Yellow Analogs.Neurochem Res. 2017 Jun;42(6):1823-1832. doi: 10.1007/s11064-017-2198-8. Epub 2017 Mar 2. Neurochem Res. 2017. PMID: 28255754
-
A new VGLUT-specific potent inhibitor: pharmacophore of Brilliant Yellow.Neurochem Res. 2014 Jan;39(1):117-28. doi: 10.1007/s11064-013-1196-8. Epub 2013 Nov 19. Neurochem Res. 2014. PMID: 24248859 Free PMC article.
-
VGLUTs in Peripheral Neurons and the Spinal Cord: Time for a Review.ISRN Neurol. 2013 Nov 20;2013:829753. doi: 10.1155/2013/829753. ISRN Neurol. 2013. PMID: 24349795 Free PMC article. Review.
-
The development of benzo- and naphtho-fused quinoline-2,4-dicarboxylic acids as vesicular glutamate transporter (VGLUT) inhibitors reveals a possible role for neuroactive steroids.Bioorg Med Chem Lett. 2014 Feb 1;24(3):850-4. doi: 10.1016/j.bmcl.2013.12.086. Epub 2013 Dec 25. Bioorg Med Chem Lett. 2014. PMID: 24424130 Free PMC article.
References
-
- Thompson CM, Davis E, Carrigan CN, Cox HD, Bridges RJ, Gerdes JM. Curr Med Chem. 2005;12:2041. - PubMed
-
- Fykse EM, Fonnum F. Neurochem Res. 1996;21:1053. - PubMed
-
- Maycox PR, Deckwerth T, Hell JW, Jahn R. J Biol Chem. 1988;263:15423. - PubMed
-
- Tabb JS, Kish PE, Van Dyke R, Ueda T. J Biol Chem. 1992;267:15412. - PubMed
-
- Wolosker H, de Souza DO, de Meis L. J Biol Chem. 1996;271:11726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

