Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by dicer-1
- PMID: 17662943
- PMCID: PMC2686109
- DOI: 10.1016/j.cell.2007.05.056
Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by dicer-1
Abstract
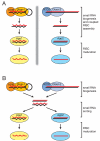
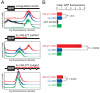
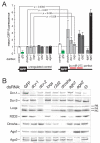
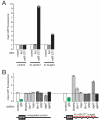
Small interfering RNAs (siRNAs) and microRNAs (miRNAs) guide distinct classes of RNA-induced silencing complexes (RISCs) to repress mRNA expression in biological processes ranging from development to antiviral defense. In Drosophila, separate but conceptually similar endonucleolytic pathways produce siRNAs and miRNAs. Here, we show that despite their distinct biogenesis, double-stranded miRNAs and siRNAs participate in a common sorting step that partitions them into Ago1- or Ago2-containing effector complexes. These distinct complexes silence their target RNAs by different mechanisms. miRNA-loaded Ago2-RISC mediates RNAi, but only Ago1 is able to repress an mRNA with central mismatches in its miRNA-binding sites. Conversely, Ago1 cannot mediate RNAi, because it is an inefficient nuclease whose catalytic rate is limited by the dissociation of its reaction products. Thus, the two members of the Drosophila Ago subclade of Argonaute proteins are functionally specialized, but specific small RNA classes are not restricted to associate with Ago1 or Ago2.
Figures


References
-
- Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. - PubMed
-
- Aza-Blanc P, Cooper CL, Wagner K, Batalov S, Deveraux QL, Cooke MP. Identification of modulators of TRAIL-induced apoptosis via RNAi-based phenotypic screening. Mol Cell. 2003;12:627–637. - PubMed
-
- Bartel DP. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell. 2004;116:281–297. - PubMed
-
- Berger EM, Dubrovsky EB, Appleby L, Dubrovskaya V. Inhibition of micro-RNA-induced RNA silencing by 2′-O-methyl oligonucleotides in Drosophila S2 cells. In Vitro Cell Dev Biol Anim. 2005;41:12–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

