Cytotoxicity of paraquat in microglial cells: Involvement of PKCdelta- and ERK1/2-dependent NADPH oxidase
- PMID: 17662968
- PMCID: PMC2084263
- DOI: 10.1016/j.brainres.2007.06.046
Cytotoxicity of paraquat in microglial cells: Involvement of PKCdelta- and ERK1/2-dependent NADPH oxidase
Abstract
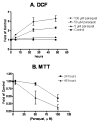
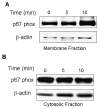
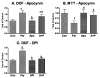
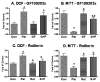
Excess production of reactive oxygen species (ROS) is an important mechanism underlying the pathogenesis of a number of neurodegenerative diseases including Parkinson's disease (PD) which is characterized by a progressive loss of dopaminergic neurons in the substantia nigra. Exposure to paraquat, an herbicide with structure similar to the dopaminergic neurotoxin, 1-methyl-4-phenylpyridinium (MPP+), has been shown to produce PD-like symptoms. Despite previous focus on the dopaminergic neurons and signaling pathways involved in their cell death, recent studies have implicated microglial cells as a major producer of ROS for damaging neighboring neurons. In this study, we examined the source of ROS and the underlying signaling pathway for paraquat-induced cytotoxicity to BV-2 microglial cells. Paraquat-induced ROS production (including superoxide anions) in BV-2 cells was accompanied by translocation of the p67phox cytosolic subunit of NADPH oxidase to the membrane. Paraquat-induced ROS production was inhibited by NADPH oxidase inhibitors, apocynin and diphenylene iodonium (DPI), but not the xanthine/xanthine oxidase inhibitor, allopurinol. Apocynin and DPI also rescued cells from paraquat-induced toxicity. The inhibitors for protein kinase C delta (PKCdelta) or extracellular signal-regulated kinases (ERK1/2) could partially attenuate paraquat-induced ROS production and cell death. Rottlerin, a selective PKCdelta inhibitor, also inhibited paraquat-induced translocation of p67phox. Taken together, this study demonstrates the involvement of ROS from NADPH oxidase in mediating paraquat cytotoxicity in BV-2 microglial cells and this process is mediated through PKCdelta- and ERK-dependent pathways.
Figures



References
-
- Andresen JJ, Faraci FM, Heistad DD. Vasomotor responses in MnSOD-deficient mice. American Journal of Physiology - Heart & Circulatory Physiology. 2004;287(3):H1141–8. - PubMed
-
- Barcia C, Bahillo AS, Fernandez-Villalba E, Bautista V, Poza MP, Fernandez-Barreiro A, Hirsch EC, Herrero M-T. Evidence of the active microglia in substantia nigra pars compacta of Parkinsonian monkeys 1 year after MPTP exposure. GLIA. 2004;46:402–9. - PubMed
-
- Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Annals of New York Academy of Sciences. 2003;991:120–31. - PubMed
-
- Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. Journal of Immunology. 2004;173:5730–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

