Evolving concepts of liver fibrogenesis provide new diagnostic and therapeutic options
- PMID: 17663771
- PMCID: PMC1994681
- DOI: 10.1186/1476-5926-6-7
Evolving concepts of liver fibrogenesis provide new diagnostic and therapeutic options
Abstract
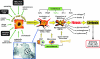
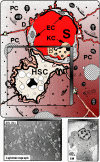
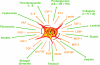
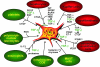
Despite intensive studies, the clinical opportunities for patients with fibrosing liver diseases have not improved. This will be changed by increasing knowledge of new pathogenetic mechanisms, which complement the "canonical principle" of fibrogenesis. The latter is based on the activation of hepatic stellate cells and their transdifferentiation to myofibroblasts induced by hepatocellular injury and consecutive inflammatory mediators such as TGF-beta. Stellate cells express a broad spectrum of matrix components. New mechanisms indicate that the heterogeneous pool of (myo-)fibroblasts can be supplemented by epithelial-mesenchymal transition (EMT) from cholangiocytes and potentially also from hepatocytes to fibroblasts, by influx of bone marrow-derived fibrocytes in the damaged liver tissue and by differentiation of a subgroup of monocytes to fibroblasts after homing in the damaged tissue. These processes are regulated by the cytokines TGF-beta and BMP-7, chemokines, colony-stimulating factors, metalloproteinases and numerous trapping proteins. They offer innovative diagnostic and therapeutic options. As an example, modulation of TGF-beta/BMP-7 ratio changes the rate of EMT, and so the simultaneous determination of these parameters and of connective tissue growth factor (CTGF) in serum might provide information on fibrogenic activity. The extension of pathogenetic concepts of fibrosis will provide new therapeutic possibilities of interference with the fibrogenic mechanism in liver and other organs.
Figures



References
-
- Schuppan D, Gressner AM. Function and metabolism of collagens and other extracellular matrix proteins. In: Bircher J, Benhamou JP, McIntyre N, Rizzetto M and Rodés J, editor. Oxford Textbook of Clinical Hepatology. 2. Vol. 2.15. Oxford, Oxford Medical Publications; 1999. pp. 381–407.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
