Functionalized fullerenes mediate photodynamic killing of cancer cells: Type I versus Type II photochemical mechanism
- PMID: 17664135
- PMCID: PMC1995806
- DOI: 10.1016/j.freeradbiomed.2007.05.005
Functionalized fullerenes mediate photodynamic killing of cancer cells: Type I versus Type II photochemical mechanism
Abstract
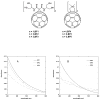
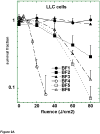
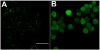
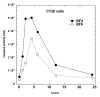
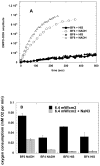
Photodynamic therapy (PDT) employs the combination of nontoxic photosensitizers (PS) and harmless visible light to generate reactive oxygen species (ROS) and kill cells. Most clinically studied PS are based on the tetrapyrrole structure of porphyrins, chlorines, and related molecules, but new nontetrapyrrole PS are being sought. Fullerenes are soccer-ball shaped molecules composed of 60 or 70 carbon atoms and have attracted interest in connection with the search for biomedical applications of nanotechnology. Fullerenes are biologically inert unless derivatized with functional groups, whereupon they become soluble and can act as PS. We have compared the photodynamic activity of six functionalized fullerenes with 1, 2, or 3 hydrophilic or 1, 2, or 3 cationic groups. The octanol-water partition coefficients were determined and the relative contributions of Type I photochemistry (photogeneration of superoxide in the presence of NADH) and Type II photochemistry (photogeneration of singlet oxygen) were studied by measurement of oxygen consumption, 1270-nm luminescence and EPR spin trapping of the superoxide product. We studied three mouse cancer cell lines: (J774, LLC, and CT26) incubated for 24 h with fullerenes and illuminated with white light. The order of effectiveness as PS was inversely proportional to the degree of substitution of the fullerene nucleus for both the neutral and the cationic series. The monopyrrolidinium fullerene was the most active PS against all cell lines and induced apoptosis 4-6 h after illumination. It produced diffuse intracellular fluorescence when dichlorodihydrofluorescein was added as an ROS probe, suggesting a Type I mechanism for phototoxicity. We conclude that certain functionalized fullerenes have potential as novel PDT agents and phototoxicity may be mediated both by superoxide and by singlet oxygen.
Figures


Similar articles
-
Photodynamic therapy with fullerenes.Photochem Photobiol Sci. 2007 Nov;6(11):1139-49. doi: 10.1039/b711141j. Epub 2007 Oct 8. Photochem Photobiol Sci. 2007. PMID: 17973044 Free PMC article. Review.
-
Photodynamic therapy with fullerenes in vivo: reality or a dream?Nanomedicine (Lond). 2011 Dec;6(10):1813-25. doi: 10.2217/nnm.11.144. Nanomedicine (Lond). 2011. PMID: 22122587 Free PMC article.
-
Cationic fullerenes are effective and selective antimicrobial photosensitizers.Chem Biol. 2005 Oct;12(10):1127-35. doi: 10.1016/j.chembiol.2005.08.014. Chem Biol. 2005. PMID: 16242655 Free PMC article.
-
Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2-* versus 1O2.J Am Chem Soc. 2003 Oct 22;125(42):12803-9. doi: 10.1021/ja0355574. J Am Chem Soc. 2003. PMID: 14558828
-
Fullerene-porphyrin nanostructures in photodynamic therapy.Nanomedicine (Lond). 2010 Feb;5(2):307-17. doi: 10.2217/nnm.09.111. Nanomedicine (Lond). 2010. PMID: 20148640 Review.
Cited by
-
Antimicrobial photodynamic therapy with fulleropyrrolidine: photoinactivation mechanism of Staphylococcus aureus, in vitro and in vivo studies.Appl Microbiol Biotechnol. 2015 May;99(9):4031-43. doi: 10.1007/s00253-015-6539-8. Epub 2015 Mar 29. Appl Microbiol Biotechnol. 2015. PMID: 25820601 Free PMC article.
-
Functionalized fullerenes in photodynamic therapy.J Biomed Nanotechnol. 2014 Sep;10(9):1918-36. doi: 10.1166/jbn.2014.1963. J Biomed Nanotechnol. 2014. PMID: 25544837 Free PMC article. Review.
-
Type I Photosensitizers Based on Aggregation-Induced Emission: A Rising Star in Photodynamic Therapy.Biosensors (Basel). 2022 Sep 4;12(9):722. doi: 10.3390/bios12090722. Biosensors (Basel). 2022. PMID: 36140107 Free PMC article. Review.
-
Mode of photoexcited C60 fullerene involvement in potentiating cisplatin toxicity against drug-resistant L1210 cells.Bioimpacts. 2019;9(4):211-217. doi: 10.15171/bi.2019.26. Epub 2019 May 22. Bioimpacts. 2019. PMID: 31799157 Free PMC article.
-
Photodynamic therapy with fullerenes.Photochem Photobiol Sci. 2007 Nov;6(11):1139-49. doi: 10.1039/b711141j. Epub 2007 Oct 8. Photochem Photobiol Sci. 2007. PMID: 17973044 Free PMC article. Review.
References
-
- Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318:162–163.
-
- Jensen AW, Wilson SR, Schuster DI. Biological applications of fullerenes. Bioorg Med Chem. 1996;4:767–779. - PubMed
-
- Bosi S, Da Ros T, Spalluto G, Prato M. Fullerene derivatives: an attractive tool for biological applications. Eur J Med Chem. 2003;38:913–923. - PubMed
-
- Triesscheijn M, Baas P, Schellens JH, Stewart FA. Photodynamic therapy in oncology. Oncologist. 2006;11:1034–1044. - PubMed
-
- Brown SB, Mellish KJ. Verteporfin: a milestone in opthalmology and photodynamic therapy. Expert Opin Pharmacother. 2001;2:351–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

