T-Cell-independent humoral immunity is sufficient for protection against fatal intracellular ehrlichia infection
- PMID: 17664264
- PMCID: PMC2044530
- DOI: 10.1128/IAI.00705-07
T-Cell-independent humoral immunity is sufficient for protection against fatal intracellular ehrlichia infection
Abstract
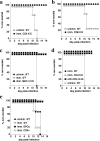
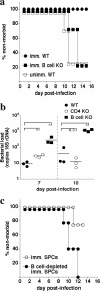
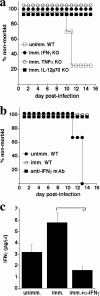
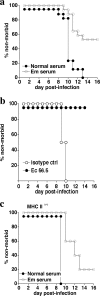
Although humoral immunity has been shown to contribute to host defense during intracellular bacterial infections, its role has generally been ancillary. Instead, CD4 T cells are often considered to play the dominant role in protective immunity via their production of type I cytokines. Our studies of highly pathogenic Ehrlichia bacteria isolated from Ixodes ovatus (IOE) reveal, however, that this paradigm is not always correct. Immunity to IOE infection can be induced by infection with a closely related weakly pathogenic ehrlichia, Ehrlichia muris. Type I cytokines (i.e., gamma interferon, tumor necrosis factor alpha, and interleukin-12) were not necessary for E. muris-induced immunity. In contrast, humoral immunity was essential, as shown by the fact that E. muris-infected B-cell-deficient mice were not protected from IOE challenge and because E. muris immunization was effective in CD4-, CD8-, and major histocompatibility complex (MHC) class II-deficient mice. Immunity was unlikely due to nonspecific inflammation, as prior infection with Listeria monocytogenes did not induce immunity to IOE. Antisera from both wild-type and MHC-II-deficient mice provided at least partial resistance to challenge infection, and protection could also be achieved following transfer of total, but not B-cell-depleted, splenocytes obtained from E. muris-immunized mice. The titers of class-switched antibodies in immunized CD4 T-cell- and MHC class II-deficient mice, although lower than those observed in immunized wild-type mice, were significant, indicating that E. muris can induce class switch recombination in the absence of classical T-cell-mediated help. These studies highlight a major protective role for classical T-cell-independent humoral immunity during an intracellular bacterial infection.
Figures


References
-
- Alugupalli, K. R., J. M. Leong, R. T. Woodland, M. Muramatsu, T. Honjo, and R. M. Gerstein. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21:379-390. - PubMed
-
- Belperron, A. A., C. M. Dailey, and L. K. Bockenstedt. 2005. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 174:5681-5686. - PubMed
-
- Bendelac, A., O. Lantz, M. E. Quimby, J. W. Yewdell, J. R. Bennick, and R. R. Brutkiewicz. 1995. CD1 recognition by mouse NK1+ T lymphocytes. Science 268:863-865. - PubMed
-
- Bitsaktsis, C., J. Huntington, and G. M. Winslow. 2004. Production of interferon-γ by CD4 T cells is essential for resolving ehrlichia infection. J. Immunol. 172:6894-6901. - PubMed
-
- Bitsaktsis, C., and G. Winslow. 2006. Fatal recall responses mediated by CD8 T cells during intracellular bacteria infection. J. Immunol. 177:4644-4651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

