Small heat shock protein alphaA-crystallin regulates epithelial sodium channel expression
- PMID: 17664274
- PMCID: PMC2361386
- DOI: 10.1074/jbc.M703409200
Small heat shock protein alphaA-crystallin regulates epithelial sodium channel expression
Abstract
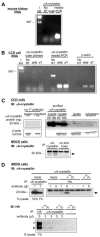
Integral membrane proteins are synthesized on the cytoplasmic face of the endoplasmic reticulum (ER). After being translocated or inserted into the ER, they fold and undergo post-translational modifications. Within the ER, proteins are also subjected to quality control checkpoints, during which misfolded proteins may be degraded by proteasomes via a process known as ER-associated degradation. Molecular chaperones, including the small heat shock protein alphaA-crystallin, have recently been shown to play a role in this process. We have now found that alphaA-crystallin is expressed in cultured mouse collecting duct cells, where apical Na(+) transport is mediated by epithelial Na(+) channels (ENaC). ENaC-mediated Na(+) currents in Xenopus oocytes were reduced by co-expression of alphaA-crystallin. This reduction in ENaC activity reflected a decrease in the number of channels expressed at the cell surface. Furthermore, we observed that the rate of ENaC delivery to the cell surface of Xenopus oocytes was significantly reduced by co-expression of alphaA-crystallin, whereas the rate of channel retrieval remained unchanged. We also observed that alphaA-crystallin and ENaC co-immunoprecipitate. These data are consistent with the hypothesis that small heat shock proteins recognize ENaC subunits at ER quality control checkpoints and can target ENaC subunits for ER-associated degradation.
Figures




References
-
- Garty H, Palmer LG. Physiol Rev. 1997;77:359–396. - PubMed
-
- Rossier BC, Pradervand S, Schild L, Hummler E. Annu Rev Physiol. 2002;64:877–897. - PubMed
-
- Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Nat Med. 2004;10:487–493. - PubMed
-
- Kosari F, Sheng S, Li J, Mak DO, Foskett JK, Kleyman TR. J Biol Chem. 1998;273:13469–13474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

