Signaling through Fc gamma RIII is required for optimal T helper type (Th)2 responses and Th2-mediated airway inflammation
- PMID: 17664287
- PMCID: PMC2118666
- DOI: 10.1084/jem.20061134
Signaling through Fc gamma RIII is required for optimal T helper type (Th)2 responses and Th2-mediated airway inflammation
Abstract
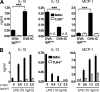
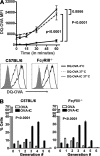
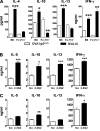
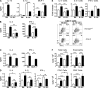
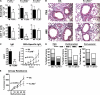
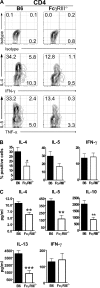
Although inhibitory Fc gamma receptors have been demonstrated to promote mucosal tolerance, the role of activating Fc gamma receptors in modulating T helper type (Th)2-dependent inflammatory responses characteristic of asthma and allergies remains unclear. Here, we demonstrate that signaling via activating Fc gamma receptors in conjunction with Toll-like receptor 4 stimulation modulated cytokine production from bone marrow-derived dendritic cells (DCs) and augmented their ability to promote Th2 responses. Ligation of the low affinity receptor Fc gamma RIII was specifically required for the enhanced Th2 responses, as Fc gamma RIII(-/-) DCs failed to augment Th2-mediated airway inflammation in vivo or induce Th2 differentiation in vitro. Further, Fc gamma RIII(-/-) mice had impaired Th2 cytokine production and exhibited reduced airway inflammation, whereas no defect was found in Fc gamma RI(-/-) mice. The augmentation of Th2 immunity was regulated by interleukin 10 production from the DCs but was distinct and independent of the well-established role of Fc gamma RIII in augmenting antigen presentation. Thus, our studies reveal a novel and specific role for Fc gamma RIII signaling in the regulation of Th cell responses and suggest that in addition to immunoglobulin (Ig)E, antigen-specific IgG also contributes to the pathogenesis of Th2-mediated diseases such as asthma and allergies.
Figures


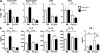
Similar articles
-
Dectin-2 promotes house dust mite-induced T helper type 2 and type 17 cell differentiation and allergic airway inflammation in mice.Am J Respir Cell Mol Biol. 2014 Aug;51(2):201-9. doi: 10.1165/rcmb.2013-0522OC. Am J Respir Cell Mol Biol. 2014. PMID: 24588637
-
Cytokine gene-modulated dendritic cells protect against allergic airway inflammation by inducing IL-10(+)IFN-gamma(+)CD4(+) T cells.Gene Ther. 2010 Aug;17(8):1011-21. doi: 10.1038/gt.2010.39. Epub 2010 Apr 1. Gene Ther. 2010. PMID: 20357831
-
Differential capacity of CD8+ alpha or CD8- alpha dendritic cell subsets to prime for eosinophilic airway inflammation in the T-helper type 2-prone milieu of the lung.Clin Exp Allergy. 2004 Dec;34(12):1834-40. doi: 10.1111/j.1365-2222.2004.02133.x. Clin Exp Allergy. 2004. PMID: 15663556
-
Mode of dendritic cell activation: the decisive hand in Th2/Th17 cell differentiation. Implications in asthma severity?Immunobiology. 2015 Feb;220(2):254-61. doi: 10.1016/j.imbio.2014.09.016. Epub 2014 Sep 16. Immunobiology. 2015. PMID: 25245013 Review.
-
Suppression of antiviral responses by antibody-dependent enhancement of macrophage infection.Trends Immunol. 2003 Apr;24(4):165-8. doi: 10.1016/s1471-4906(03)00065-6. Trends Immunol. 2003. PMID: 12697441 Review.
Cited by
-
Eosinophil-derived chemokine (hCCL15/23, mCCL6) interacts with CCR1 to promote eosinophilic airway inflammation.Signal Transduct Target Ther. 2021 Feb 28;6(1):91. doi: 10.1038/s41392-021-00482-x. Signal Transduct Target Ther. 2021. PMID: 33640900 Free PMC article.
-
Programming dendritic cells to induce T(H)2 and tolerogenic responses.Nat Immunol. 2010 Aug;11(8):647-55. doi: 10.1038/ni.1894. Epub 2010 Jul 20. Nat Immunol. 2010. PMID: 20644570 Review.
-
Interleukin-22 attenuates allergic airway inflammation in ovalbumin-induced asthma mouse model.BMC Pulm Med. 2021 Nov 26;21(1):385. doi: 10.1186/s12890-021-01698-x. BMC Pulm Med. 2021. PMID: 34836520 Free PMC article.
-
Inhibition of Pyk2 blocks airway inflammation and hyperresponsiveness in a mouse model of asthma.Am J Respir Cell Mol Biol. 2010 Apr;42(4):491-7. doi: 10.1165/rcmb.2008-0469OC. Epub 2009 Jun 11. Am J Respir Cell Mol Biol. 2010. PMID: 19520918 Free PMC article.
-
Eosinophil-derived CCL-6 impairs hematopoietic stem cell homeostasis.Cell Res. 2018 Mar;28(3):323-335. doi: 10.1038/cr.2018.2. Epub 2018 Jan 12. Cell Res. 2018. PMID: 29327730 Free PMC article.
References
-
- Bochner, B.S., and W.W. Busse. 2005. Allergy and asthma. J. Allergy Clin. Immunol. 115:953–959. - PubMed
-
- van Rijt, L.S., and B.N. Lambrecht. 2001. Role of dendritic cells and Th2 lymphocytes in asthma: lessons from eosinophilic airway inflammation in the mouse. Microsc. Res. Tech. 53:256–272. - PubMed
-
- Bruhns, P., S. Fremont, and M. Daeron. 2005. Regulation of allergy by Fc receptors. Curr. Opin. Immunol. 17:662–669. - PubMed
-
- Grove, D.I., J.G. Reid, and I.J. Forbes. 1975. Humoral and cellular immunity in atopic eczema. Br. J. Dermatol. 92:611–618. - PubMed
-
- Pepys, J., W.E. Parish, B. Stenius-Aarniala, and L. Wide. 1979. Clinical correlations between long-term (IgE) and short-term (IgG S-TS) anaphylactic antibodies in atopic and ‘non-atopic’ subjects with respiratory allergic disease. Clin. Allergy. 9:645–658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

