Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease
- PMID: 17664291
- PMCID: PMC2118680
- DOI: 10.1084/jem.20070304
Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease
Abstract
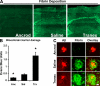
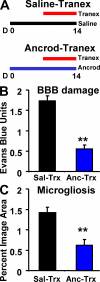
Cerebrovascular dysfunction contributes to the pathology and progression of Alzheimer's disease (AD), but the mechanisms are not completely understood. Using transgenic mouse models of AD (TgCRND8, PDAPP, and Tg2576), we evaluated blood-brain barrier damage and the role of fibrin and fibrinolysis in the progression of amyloid-beta pathology. These mouse models showed age-dependent fibrin deposition coincident with areas of blood-brain barrier permeability as demonstrated by Evans blue extravasation. Three lines of evidence suggest that fibrin contributes to the pathology. First, AD mice with only one functional plasminogen gene, and therefore with reduced fibrinolysis, have increased neurovascular damage relative to AD mice. Conversely, AD mice with only one functional fibrinogen gene have decreased blood-brain barrier damage. Second, treatment of AD mice with the plasmin inhibitor tranexamic acid aggravated pathology, whereas removal of fibrinogen from the circulation of AD mice with ancrod treatment attenuated measures of neuroinflammation and vascular pathology. Third, pretreatment with ancrod reduced the increased pathology from plasmin inhibition. These results suggest that fibrin is a mediator of inflammation and may impede the reparative process for neurovascular damage in AD. Fibrin and the mechanisms involved in its accumulation and clearance may present novel therapeutic targets in slowing the progression of AD.
Figures


Similar articles
-
Fibrin deposited in the Alzheimer's disease brain promotes neuronal degeneration.Neurobiol Aging. 2015 Feb;36(2):608-17. doi: 10.1016/j.neurobiolaging.2014.10.030. Epub 2014 Oct 31. Neurobiol Aging. 2015. PMID: 25475538 Free PMC article.
-
Fibrinogen and altered hemostasis in Alzheimer's disease.J Alzheimers Dis. 2012;32(3):599-608. doi: 10.3233/JAD-2012-120820. J Alzheimers Dis. 2012. PMID: 22869464 Free PMC article. Review.
-
Long-Term Dabigatran Treatment Delays Alzheimer's Disease Pathogenesis in the TgCRND8 Mouse Model.J Am Coll Cardiol. 2019 Oct 15;74(15):1910-1923. doi: 10.1016/j.jacc.2019.07.081. J Am Coll Cardiol. 2019. PMID: 31601371 Free PMC article.
-
Blood-derived plasminogen drives brain inflammation and plaque deposition in a mouse model of Alzheimer's disease.Proc Natl Acad Sci U S A. 2018 Oct 9;115(41):E9687-E9696. doi: 10.1073/pnas.1811172115. Epub 2018 Sep 25. Proc Natl Acad Sci U S A. 2018. PMID: 30254165 Free PMC article.
-
A Leaky Blood-Brain Barrier to Fibrinogen Contributes to Oxidative Damage in Alzheimer's Disease.Antioxidants (Basel). 2021 Dec 31;11(1):102. doi: 10.3390/antiox11010102. Antioxidants (Basel). 2021. PMID: 35052606 Free PMC article. Review.
Cited by
-
Upregulation of glycolytic enzymes, mitochondrial dysfunction and increased cytotoxicity in glial cells treated with Alzheimer's disease plasma.PLoS One. 2015 Mar 18;10(3):e0116092. doi: 10.1371/journal.pone.0116092. eCollection 2015. PLoS One. 2015. PMID: 25785936 Free PMC article.
-
Cellular and molecular mechanisms of the blood-brain barrier dysfunction in neurodegenerative diseases.Fluids Barriers CNS. 2024 Jul 19;21(1):60. doi: 10.1186/s12987-024-00557-1. Fluids Barriers CNS. 2024. PMID: 39030617 Free PMC article. Review.
-
Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging.Neuron. 2010 Nov 4;68(3):409-27. doi: 10.1016/j.neuron.2010.09.043. Neuron. 2010. PMID: 21040844 Free PMC article.
-
High-sucrose diets contribute to brain angiopathy with impaired glucose uptake and psychosis-related higher brain dysfunctions in mice.Sci Adv. 2021 Nov 12;7(46):eabl6077. doi: 10.1126/sciadv.abl6077. Epub 2021 Nov 10. Sci Adv. 2021. PMID: 34757783 Free PMC article.
-
Eradication of Helicobacter pylori Is Associated with the Progression of Dementia: A Population-Based Study.Gastroenterol Res Pract. 2013;2013:175729. doi: 10.1155/2013/175729. Epub 2013 Nov 25. Gastroenterol Res Pract. 2013. PMID: 24371435 Free PMC article.
References
-
- Hardy, J., and D.J. Selkoe. 2002. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 297:353–356. - PubMed
-
- de la Torre, J.C. 2004. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 3:184–190. - PubMed
-
- Farkas, E., and P.G. Luiten. 2001. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog. Neurobiol. 64:575–611. - PubMed
-
- Vinters, H.V., Z.Z. Wang, and D.L. Secor. 1996. Brain parenchymal and microvascular amyloid in Alzheimer's disease. Brain Pathol. 6:179–195. - PubMed
-
- Fiala, M., Q.N. Liu, J. Sayre, V. Pop, V. Brahmandam, M.C. Graves, and H.V. Vinters. 2002. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer's disease brain and damage the blood-brain barrier. Eur. J. Clin. Invest. 32:360–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

