The neurite outgrowth multiadaptor RhoGAP, NOMA-GAP, regulates neurite extension through SHP2 and Cdc42
- PMID: 17664338
- PMCID: PMC2064841
- DOI: 10.1083/jcb.200609146
The neurite outgrowth multiadaptor RhoGAP, NOMA-GAP, regulates neurite extension through SHP2 and Cdc42
Abstract
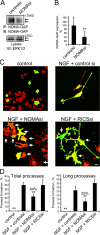
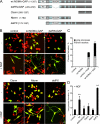
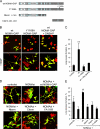
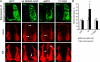
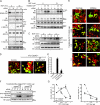
Neuronal differentiation involves the formation and extension of neuronal processes. We have identified a novel regulator of neurite formation and extension, the neurite outgrowth multiadaptor, NOMA-GAP, which belongs to a new family of multiadaptor proteins with RhoGAP activity. We show that NOMA-GAP is essential for NGF-stimulated neuronal differentiation and for the regulation of the ERK5 MAP kinase and the Cdc42 signaling pathways downstream of NGF. NOMA-GAP binds directly to the NGF receptor, TrkA, and becomes tyrosine phosphorylated upon receptor activation, thus enabling recruitment and activation of the tyrosine phosphatase SHP2. Recruitment of SHP2 is required for the stimulation of neuronal process extension and for sustained activation of ERK5 downstream of NOMA-GAP. In addition, we show that NOMA-GAP promotes neurite outgrowth by tempering activation of the Cdc42/PAK signaling pathway in response to NGF. NOMA-GAP, through its dual function as a multiadaptor and RhoGAP protein, thus plays an essential role downstream of NGF in promoting neurite outgrowth and extension.
Figures



References
-
- Allen, M.J., X. Shan, and R.K. Murphey. 2000. A role for Drosophila Drac1 in neurite outgrowth and synaptogenesis in the giant fiber system. Mol. Cell. Neurosci. 16:754–765. - PubMed
-
- Aoki, K., T. Nakamura, and M. Matsuda. 2004. Spatio-temporal regulation of Rac1 and Cdc42 activity during nerve growth factor-induced neurite outgrowth in PC12 cells. J. Biol. Chem. 279:713–719. - PubMed
-
- Arevalo, J.C., D.B. Pereira, H. Yano, K.K. Teng, and M.V. Chao. 2006. Identification of a switch in neurotrophin signaling by selective tyrosine phosphorylation. J. Biol. Chem. 281:1001–1007. - PubMed
-
- Bentires-Alj, M., J.G. Paez, F.S. David, H. Keilhack, B. Halmos, K. Naoki, J.M. Maris, A. Richardson, A. Bardelli, D.J. Sugarbaker, et al. 2004. Activating mutations of the Noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 64:8816–8820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

