Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell cell contacts in mammalian epithelial cells
- PMID: 17664339
- PMCID: PMC2064848
- DOI: 10.1083/jcb.200612042
Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell cell contacts in mammalian epithelial cells
Abstract
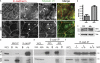
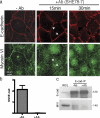
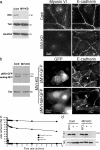
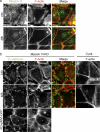
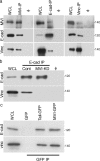
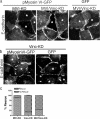
Cooperation between cadherins and the actin cytoskeleton controls many aspects of epithelial biogenesis. We report here that myosin VI critically regulates the morphogenesis of epithelial cell-cell contacts. As epithelial monolayers mature in culture, discontinuous cell-cell contacts are initially replaced by continuous (cohesive) contacts. Myosin VI is recruited to cell contacts as they become linear and cohesive, where it forms a biochemical complex with epithelial cadherin (E-cadherin). Myosin VI is necessary for strong cadherin adhesion, for cells to form cohesive linear contacts, and for the integrity of the apical junctional complex. We find that vinculin mediates this effect of myosin VI. Myosin VI is necessary for vinculin and E-cadherin to interact. A combination of gain and loss of function approaches identifies vinculin as a downstream effector of myosin VI that is necessary for the integrity of intercellular contacts. We propose that myosin VI and vinculin form a molecular apparatus that generates cohesive cell-cell contacts in cultured mammalian epithelia.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

