Intracellular Ca2+ oscillations, a potential pacemaking mechanism in early embryonic heart cells
- PMID: 17664344
- PMCID: PMC2151640
- DOI: 10.1085/jgp.200609575
Intracellular Ca2+ oscillations, a potential pacemaking mechanism in early embryonic heart cells
Abstract
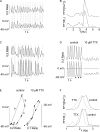
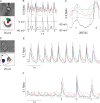
Early (E9.5-E11.5) embryonic heart cells beat spontaneously, even though the adult pacemaking mechanisms are not yet fully established. Here we show that in isolated murine early embryonic cardiomyocytes periodic oscillations of cytosolic Ca(2+) occur and that these induce contractions. The Ca(2+) oscillations originate from the sarcoplasmic reticulum and are dependent on the IP(3) and the ryanodine receptor. The Ca(2+) oscillations activate the Na(+)-Ca(2+) exchanger, giving rise to subthreshold depolarizations of the membrane potential and/or action potentials. Although early embryonic heart cells are voltage-independent Ca(2+) oscillators, the generation of action potentials provides synchronization of the electrical and mechanical signals. Thus, Ca(2+) oscillations pace early embryonic heart cells and the ensuing activation of the Na(+)-Ca(2+) exchanger evokes small membrane depolarizations or action potentials.
Figures
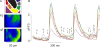




References
-
- Baruscotti, M., and D. DiFrancesco. 2004. Pacemaker channels. Ann. N. Y. Acad. Sci. 1015:111–121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

