Cytoplasmic ATP inhibition of CLC-1 is enhanced by low pH
- PMID: 17664348
- PMCID: PMC2151639
- DOI: 10.1085/jgp.200709817
Cytoplasmic ATP inhibition of CLC-1 is enhanced by low pH
Abstract
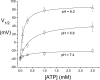
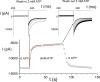
The CLC-1 Cl(-) channel is abundantly expressed on the plasma membrane of muscle cells, and the membrane potential of muscle cells is largely controlled by the activity of this Cl(-) channel. Previous studies showed that low intracellular pH increases the overall open probability of recombinant CLC-1 channels in various expression systems. Low intracellular pH, however, is known to inhibit the Cl(-) conductance on the native muscle membrane, contradicting the findings from the recombinant CLC-1 channels in expressed systems. Here we show that in the presence of physiological concentrations of ATP, reduction of the intracellular pH indeed inhibits the expressed CLC-1, mostly by decreasing the open probability of the common gate of the channel.
Figures


Comment in
-
To ATP or not to ATP: this is the question.J Gen Physiol. 2008 Feb;131(2):105-8. doi: 10.1085/jgp.200709953. J Gen Physiol. 2008. PMID: 18227270 Free PMC article. No abstract available.
References
-
- Bennetts, B., G.Y. Rychkov, H.L. Ng, C.J. Morton, D. Stapleton, M.W. Parker, and B.A. Cromer. 2005. Cytoplasmic ATP-sensing domains regulate gating of skeletal muscle ClC-1 chloride channels. J. Biol. Chem. 280:32452–32458. - PubMed

