Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles
- PMID: 17664416
- PMCID: PMC1941812
- DOI: 10.1073/pnas.0705947104
Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles
Abstract
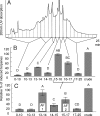
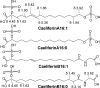
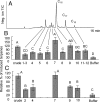
A previously unidentified class of compounds has been isolated from the regurgitant of the grasshopper species Schistocerca americana. These compounds (named here "caeliferins") are composed of saturated and monounsaturated sulfated alpha-hydroxy fatty acids in which the omega-carbon is functionalized with either a sulfated hydroxyl or a carboxyl conjugated to glycine via an amide bond. The regurgitant contains a series of these compounds with fatty acid chains of 15-20 carbons and in varying proportions. Of these, the 16-carbon analogs are predominant and are also most active in inducing release of volatile organic compounds when applied to damaged leaves of corn seedlings. Caeliferins are nonlepidopteran elicitors identified in insect herbivores. This adds a category of insect herbivore-produced elicitors of plant responses, providing further evidence of the ability of plants to detect and respond to a broad range of insect herbivore-produced compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Airborne signals prime plants against insect herbivore attack.Proc Natl Acad Sci U S A. 2004 Feb 10;101(6):1781-5. doi: 10.1073/pnas.0308037100. Epub 2004 Jan 28. Proc Natl Acad Sci U S A. 2004. PMID: 14749516 Free PMC article.
-
Insect elicitors and exposure to green leafy volatiles differentially upregulate major octadecanoids and transcripts of 12-oxo phytodienoic acid reductases in Zea mays.Mol Plant Microbe Interact. 2007 Jun;20(6):707-16. doi: 10.1094/MPMI-20-6-0707. Mol Plant Microbe Interact. 2007. PMID: 17555278
-
Differential activity and degradation of plant volatile elicitors in regurgitant of tobacco hornworm (Manduca sexta) larvae.J Chem Ecol. 2003 Jun;29(6):1357-72. doi: 10.1023/a:1024209302628. J Chem Ecol. 2003. PMID: 12918921
-
Biosynthesis of fatty acid amide elicitors of plant volatiles by insect herbivores.Arch Insect Biochem Physiol. 2005 Feb;58(2):54-68. doi: 10.1002/arch.20036. Arch Insect Biochem Physiol. 2005. PMID: 15660361 Review.
-
Plant production of volatile semiochemicals in response to insect-derived elicitors.Novartis Found Symp. 1999;223:95-105; discussion 105-9, 160-5. doi: 10.1002/9780470515679.ch7. Novartis Found Symp. 1999. PMID: 10549550 Review.
Cited by
-
Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice.Proc Natl Acad Sci U S A. 2009 Dec 29;106(52):22163-8. doi: 10.1073/pnas.0912139106. Epub 2009 Dec 14. Proc Natl Acad Sci U S A. 2009. PMID: 20018701 Free PMC article.
-
Characterization and Function of 3-Hydroxy-3-Methylglutaryl-CoA Reductase in Populus trichocarpa: Overexpression of PtHMGR Enhances Terpenoids in Transgenic Poplar.Front Plant Sci. 2019 Nov 15;10:1476. doi: 10.3389/fpls.2019.01476. eCollection 2019. Front Plant Sci. 2019. PMID: 31803212 Free PMC article.
-
SmCSP4 from aphid saliva stimulates salicylic acid-mediated defence responses in wheat by interacting with transcription factor TaWKRY76.Plant Biotechnol J. 2023 Nov;21(11):2389-2407. doi: 10.1111/pbi.14139. Epub 2023 Aug 4. Plant Biotechnol J. 2023. PMID: 37540474 Free PMC article.
-
Carrageenans, sulphated polysaccharides of red seaweeds, differentially affect Arabidopsis thaliana resistance to Trichoplusia ni (cabbage looper).PLoS One. 2011;6(10):e26834. doi: 10.1371/journal.pone.0026834. Epub 2011 Oct 28. PLoS One. 2011. PMID: 22046375 Free PMC article.
-
MAPK signaling: a key element in plant defense response to insects.Insect Sci. 2015 Apr;22(2):157-64. doi: 10.1111/1744-7917.12128. Epub 2014 Aug 8. Insect Sci. 2015. PMID: 24753304 Free PMC article. Review.
References
-
- Turlings TCJ, Tumlinson JH, Lewis WJ. Science. 1990;250:1251–1253. - PubMed
-
- Kessler A, Baldwin IT. Science. 2001;291:2141–2144. - PubMed
-
- Loughrin JH, Manukian A, Heath RR, Tumlinson JH. J Chem Ecol. 1995:1217–1227. - PubMed
-
- Gouinguene S, Degen T, Turlings TCJ. Chemoecol. 2001;11:9–16.
-
- Paré PW, Tumlinson JH. Nature. 1997;385:30–31.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

