Assays of vacuole fusion resolve the stages of docking, lipid mixing, and content mixing
- PMID: 17664431
- PMCID: PMC1941832
- DOI: 10.1073/pnas.0700970104
Assays of vacuole fusion resolve the stages of docking, lipid mixing, and content mixing
Abstract
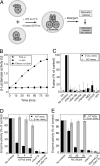
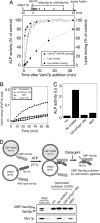
Membrane fusion entails organelle docking and subsequent mixing of membrane bilayers and luminal compartments. We now present an in vitro assay of fusion, using yeast vacuoles bearing domains of either Fos or Jun fused to complementary halves of beta-lactamase. Upon fusion, these proteins associate to yield beta-lactamase activity. This assay complements the standard fusion assay (activation of pro-Pho8p in protease-deficient vacuoles by proteases from pho8Delta vacuoles). Both the beta-lactamase and pro-Pho8p activation assays of fusion show the same long kinetic delay between SNARE pairing and luminal compartment mixing. Lipid-mixing occurs rapidly after SNARE pairing but well before aqueous compartment mixing. These results support a model in which SNARE pairing leads to rapid hemifusion, followed by slow further lipid rearrangement and aqueous compartment mixing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Transition from hemifusion to pore opening is rate limiting for vacuole membrane fusion.J Cell Biol. 2005 Dec 19;171(6):981-90. doi: 10.1083/jcb.200510018. J Cell Biol. 2005. PMID: 16365164 Free PMC article.
-
Trans-SNARE pairing can precede a hemifusion intermediate in intracellular membrane fusion.Nature. 2005 Jul 21;436(7049):410-4. doi: 10.1038/nature03722. Epub 2005 May 29. Nature. 2005. PMID: 15924133
-
Ergosterol is required for the Sec18/ATP-dependent priming step of homotypic vacuole fusion.EMBO J. 2001 Aug 1;20(15):4035-40. doi: 10.1093/emboj/20.15.4035. EMBO J. 2001. PMID: 11483507 Free PMC article.
-
Yeast homotypic vacuole fusion: a window on organelle trafficking mechanisms.Annu Rev Biochem. 2000;69:247-75. doi: 10.1146/annurev.biochem.69.1.247. Annu Rev Biochem. 2000. PMID: 10966459 Review.
-
Interplay between lipids and the proteinaceous membrane fusion machinery.Prog Lipid Res. 2008 Nov;47(6):461-9. doi: 10.1016/j.plipres.2008.08.002. Epub 2008 Sep 19. Prog Lipid Res. 2008. PMID: 18805437 Review.
Cited by
-
Sec18p and Vam7p remodel trans-SNARE complexes to permit a lipid-anchored R-SNARE to support yeast vacuole fusion.EMBO J. 2007 Dec 12;26(24):4935-45. doi: 10.1038/sj.emboj.7601915. Epub 2007 Nov 15. EMBO J. 2007. PMID: 18007597 Free PMC article.
-
Sphingolipids containing very long-chain fatty acids regulate Ypt7 function during the tethering stage of vacuole fusion.J Biol Chem. 2024 Nov;300(11):107808. doi: 10.1016/j.jbc.2024.107808. Epub 2024 Sep 21. J Biol Chem. 2024. PMID: 39307308 Free PMC article.
-
Vibrio effector protein VopQ inhibits fusion of V-ATPase-containing membranes.Proc Natl Acad Sci U S A. 2015 Jan 6;112(1):100-5. doi: 10.1073/pnas.1413764111. Epub 2014 Dec 1. Proc Natl Acad Sci U S A. 2015. PMID: 25453092 Free PMC article.
-
Fusion step-specific influence of cholesterol on SNARE-mediated membrane fusion.Biophys J. 2009 Mar 4;96(5):1839-46. doi: 10.1016/j.bpj.2008.11.033. Biophys J. 2009. PMID: 19254542 Free PMC article.
-
Molecular mechanism of the synaptotagmin-SNARE interaction in Ca2+-triggered vesicle fusion.Nat Struct Mol Biol. 2010 Mar;17(3):325-31. doi: 10.1038/nsmb.1764. Epub 2010 Feb 21. Nat Struct Mol Biol. 2010. PMID: 20173762 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

