Single-molecule fluorescence spectroscopy in (bio)catalysis
- PMID: 17664433
- PMCID: PMC1937513
- DOI: 10.1073/pnas.0610755104
Single-molecule fluorescence spectroscopy in (bio)catalysis
Abstract
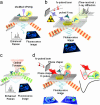
The ever-improving time and space resolution and molecular detection sensitivity of fluorescence microscopy offer unique opportunities to deepen our insights into the function of chemical and biological catalysts. Because single-molecule microscopy allows for counting the turnover events one by one, one can map the distribution of the catalytic activities of different sites in solid heterogeneous catalysts, or one can study time-dependent activity fluctuations of individual sites in enzymes or chemical catalysts. By experimentally monitoring individuals rather than populations, the origin of complex behavior, e.g., in kinetics or in deactivation processes, can be successfully elucidated. Recent progress of temporal and spatial resolution in single-molecule fluorescence microscopy is discussed in light of its impact on catalytic assays. Key concepts are illustrated regarding the use of fluorescent reporters in catalytic reactions. Future challenges comprising the integration of other techniques, such as diffraction, scanning probe, or vibrational methods in single-molecule fluorescence spectroscopy are suggested.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Single molecule methods for the study of catalysis: from enzymes to heterogeneous catalysts.Chem Soc Rev. 2014 Feb 21;43(4):990-1006. doi: 10.1039/c3cs60245a. Chem Soc Rev. 2014. PMID: 24085063 Review.
-
Quantifying intracellular dynamics using fluorescence fluctuation spectroscopy.Protoplasma. 2014 Mar;251(2):307-16. doi: 10.1007/s00709-013-0602-z. Epub 2014 Jan 14. Protoplasma. 2014. PMID: 24420265 Review.
-
Spatially resolved observation of crystal-face-dependent catalysis by single turnover counting.Nature. 2006 Feb 2;439(7076):572-5. doi: 10.1038/nature04502. Nature. 2006. PMID: 16452976
-
Fluorescence fluctuation spectroscopy in reduced detection volumes.Curr Pharm Biotechnol. 2006 Feb;7(1):51-66. doi: 10.2174/138920106775789629. Curr Pharm Biotechnol. 2006. PMID: 16472133 Review.
-
Sorting single molecules: application to diagnostics and evolutionary biotechnology.Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5740-7. doi: 10.1073/pnas.91.13.5740. Proc Natl Acad Sci U S A. 1994. PMID: 7517036 Free PMC article. Review.
Cited by
-
Single-molecule photoredox catalysis.Chem Sci. 2018 Oct 22;10(3):681-687. doi: 10.1039/c8sc03860k. eCollection 2019 Jan 21. Chem Sci. 2018. PMID: 30746104 Free PMC article.
-
Amalgamation of DNAzymes and Nanozymes in a Coronazyme.J Am Chem Soc. 2023 Mar 15;145(10):5750-5758. doi: 10.1021/jacs.2c12367. Epub 2023 Feb 16. J Am Chem Soc. 2023. PMID: 36795472 Free PMC article.
-
Heterogeneities of individual catalyst particles in space and time as monitored by spectroscopy.Nat Chem. 2012 Nov;4(11):873-86. doi: 10.1038/nchem.1478. Epub 2012 Oct 23. Nat Chem. 2012. PMID: 23089861 Review.
-
Rationalizing inter- and intracrystal heterogeneities in dealuminated acid mordenite zeolites by stimulated Raman scattering microscopy correlated with super-resolution fluorescence microscopy.ACS Nano. 2014 Dec 23;8(12):12650-9. doi: 10.1021/nn505576p. Epub 2014 Nov 20. ACS Nano. 2014. PMID: 25402756 Free PMC article.
-
Computationally Efficient Modelling of Stochastic Spatio-Temporal Dynamics in Biomolecular Networks.Sci Rep. 2018 Feb 22;8(1):3498. doi: 10.1038/s41598-018-21826-8. Sci Rep. 2018. PMID: 29472589 Free PMC article.
References
-
- de Levie R. J Chem Educ. 2000;77:771–774.
-
- Xie XS, Lu HP. J Biol Chem. 1999;274:15967–15970. - PubMed
-
- Engelkamp H, Hatzakis NS, Hofkens J, De Schryver FC, Nolte RJM, Rowan AE. Chem Commun. 2006;9:935–940. - PubMed
-
- Smiley RD, Hammes GG. Chem Rev. 2006;106:3080–3094. - PubMed
-
- Craig DB, Arriaga EA, Wong JCY, Lu H, Dovichi NJ. J Am Chem Soc. 1996;118:5245–5253.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

