Selective induction of apoptosis in leukemic B-lymphoid cells by a CD19-specific TRAIL fusion protein
- PMID: 17665197
- PMCID: PMC11030665
- DOI: 10.1007/s00262-007-0370-8
Selective induction of apoptosis in leukemic B-lymphoid cells by a CD19-specific TRAIL fusion protein
Abstract
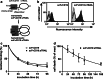
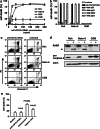
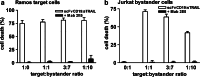
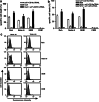
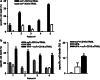
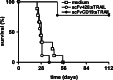
Although the treatment outcome of lymphoid malignancies has improved in recent years by the introduction of transplantation and antibody-based therapeutics, relapse remains a major problem. Therefore, new therapeutic options are urgently needed. One promising approach is the selective activation of apoptosis in tumor cells by the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). This study investigated the pro-apoptotic potential of a novel TRAIL fusion protein designated scFvCD19:sTRAIL, consisting of a CD19-specific single-chain Fv antibody fragment (scFv) fused to the soluble extracellular domain of TRAIL (sTRAIL). Potent apoptosis was induced by scFvCD19:sTRAIL in several CD19-positive tumor cell lines, whereas normal blood cells remained unaffected. In mixed culture experiments, selective binding of scFvCD19:sTRAIL to CD19-positive cells resulted in strong induction of apoptosis in CD19-negative bystander tumor cells. Simultaneous treatment of CD19-positive cell lines with scFvCD19:sTRAIL and valproic acid (VPA) or Cyclosporin A induced strongly synergistic apoptosis. Treatment of patient-derived acute B-lymphoblastic leukemia (B-ALL) and chronic B-lymphocytic leukemia (B-CLL) cells resulted in strong tumoricidal activity that was further enhanced by combination with VPA. In addition, scFvCD19:sTRAIL prevented engraftment of human Nalm-6 cells in xenotransplanted NOD/Scid mice. The pre-clinical data presented here warrant further investigation of scFvCD19:sTRAIL as a potential new therapeutic agent for CD19-positive B-lineage malignancies.
Figures
References
-
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155. doi: 10.1172/JCI6926. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

