Timed interactions between viral and cellular replication factors during the initiation of SV40 in vitro DNA replication
- PMID: 17666013
- PMCID: PMC2049014
- DOI: 10.1042/BJ20070794
Timed interactions between viral and cellular replication factors during the initiation of SV40 in vitro DNA replication
Abstract
The initiation of SV40 (simian virus 40) DNA replication requires the co-operative interactions between the viral Tag (large T-antigen), RPA (replication protein A) and Pol (DNA polymerase alpha-primase) on the template DNA. Binding interfaces mapped on these enzymes and expressed as peptides competed with the mutual interactions of the native proteins. Prevention of the genuine interactions was accomplished only prior to the primer synthesis step and blocked the assembly of a productive initiation complex. Once the complex was engaged in the synthesis of an RNA primer and its extension, the interfering effects of the peptides ceased, suggesting a stable association of the replication factors during the initiation phase. Specific antibodies were still able to disrupt preformed interactions and inhibited primer synthesis and extension activities, underlining the crucial role of specific protein-protein contacts during the entire initiation process.
Figures

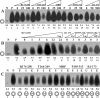


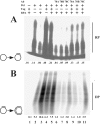
Similar articles
-
Different activities of the largest subunit of replication protein A cooperate during SV40 DNA replication.FEBS Lett. 2007 Aug 21;581(21):3973-8. doi: 10.1016/j.febslet.2007.07.038. Epub 2007 Jul 25. FEBS Lett. 2007. PMID: 17673209 Free PMC article.
-
Mutational analysis of simian virus 40 T-antigen primosome activities in viral DNA replication.J Virol. 2002 May;76(10):5121-30. doi: 10.1128/jvi.76.10.5121-5130.2002. J Virol. 2002. PMID: 11967327 Free PMC article.
-
The replication protein A binding site in simian virus 40 (SV40) T antigen and its role in the initial steps of SV40 DNA replication.J Virol. 1998 Dec;72(12):9771-81. doi: 10.1128/JVI.72.12.9771-9781.1998. J Virol. 1998. PMID: 9811712 Free PMC article.
-
The initiation of simian virus 40 DNA replication in vitro.Crit Rev Biochem Mol Biol. 1997;32(6):503-68. doi: 10.3109/10409239709082001. Crit Rev Biochem Mol Biol. 1997. PMID: 9444478 Review.
-
DNA replication and the cell cycle.Ciba Found Symp. 1992;170:147-56; discussion 156-60. doi: 10.1002/9780470514320.ch10. Ciba Found Symp. 1992. PMID: 1336449 Review.
Cited by
-
Host-specific replication of BK virus DNA in mouse cell extracts is independently controlled by DNA polymerase alpha-primase and inhibitory activities.J Virol. 2010 Jul;84(13):6636-44. doi: 10.1128/JVI.00527-10. Epub 2010 Apr 14. J Virol. 2010. PMID: 20392840 Free PMC article.
-
Restriction of human polyomavirus BK virus DNA replication in murine cells and extracts.J Virol. 2009 Jun;83(11):5708-17. doi: 10.1128/JVI.00300-09. Epub 2009 Mar 18. J Virol. 2009. PMID: 19297467 Free PMC article.
-
Functions of alternative replication protein A in initiation and elongation.Biochemistry. 2010 Jul 20;49(28):5919-28. doi: 10.1021/bi100380n. Biochemistry. 2010. PMID: 20545304 Free PMC article.
-
Physical interactions between Mcm10, DNA, and DNA polymerase alpha.J Biol Chem. 2009 Sep 4;284(36):24662-72. doi: 10.1074/jbc.M109.020438. Epub 2009 Jul 16. J Biol Chem. 2009. PMID: 19608746 Free PMC article.
References
-
- Kelly T. J. SV40 DNA replication. J. Biol. Chem. 1988;263:17889–17892. - PubMed
-
- Stenlund A. Initiation of DNA replication: lessons from viral initiator proteins. Nat. Rev. Mol. Cell Biol. 2003;4:777–785. - PubMed
-
- Simmons D. T. SV40 large T antigen functions in DNA replication and transformation. Adv. Virus Res. 2000;55:75–134. - PubMed
-
- Waga S., Bauer G., Stillman B. Reconstitution of complete SV40 DNA replication with purified replication factors. J. Biol. Chem. 1994;269:10923–10934. - PubMed
-
- Stauffer M. E., Chazin W. J. Structural mechanisms of DNA replication, repair, and recombination. J. Biol. Chem. 2004;279:30915–30918. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

