Formation of specialized propagules resistant to desiccation and cryopreservation in the threatened moss Ditrichum plumbicola (Ditrichales, Bryopsida)
- PMID: 17666410
- PMCID: PMC2533608
- DOI: 10.1093/aob/mcm141
Formation of specialized propagules resistant to desiccation and cryopreservation in the threatened moss Ditrichum plumbicola (Ditrichales, Bryopsida)
Abstract
Background and aims: Successful cryopreservation of bryophytes is linked to intrinsic desiccation tolerance and survival can be enhanced by pre-treatment with abscisic acid (ABA) and sucrose. The pioneer moss Ditrichum plumbicola is naturally subjected to desiccation in the field but showed unexpectedly low survival of cryopreservation, as well as a poor response to pre-treatment. The effects of the cryopreservation protocol on protonemata of D. plumbicola were investigated in order to explore possible relationships between the production in vitro of cryopreservation-tolerant asexual propagules and the reproductive biology of D. plumbicola in nature.
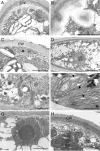
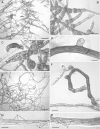
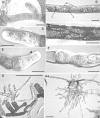
Methods: Protonemata were prepared for cryopreservation using a four-step protocol involving encapsulation in sodium alginate, pre-treatment for 2 weeks with ABA and sucrose, desiccation for 6 h and rapid freezing in liquid nitrogen. After each stage, protonemata were prepared for light and electron microscopy and growth on standard medium was monitored. Further samples were prepared for light and electron microscopy at intervals over a 24-h period following removal from liquid nitrogen and re-hydration.
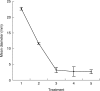
Key results: Pre-treatment with ABA and sucrose caused dramatic changes to the protonemata. Growth was arrested and propagules induced with pronounced morphological and cytological changes. Most cells died, but those that survived were characterized by thick, deeply pigmented walls, numerous small vacuoles and lipid droplets in their cytoplasm. Desiccation and cryopreservation elicited no dramatic cytological changes. Cells returned to their pre-dehydration and cryopreservation state within 2 h of re-hydration and/or removal from liquid nitrogen. Regeneration was normal once the ABA/sucrose stimulus was removed.
Conclusions: The ABA/sucrose pre-treatment induced the formation of highly desiccation- and cryopreservation-tolerant propagules from the protonemata of D. plumbicola. This parallels behaviour in the wild, where highly desiccation-tolerant rhizoids function as perennating organs allowing the moss to endure extreme environmental conditions. An involvement of endogenous ABA in the desiccation tolerance of D. plumbicola is suggested.
Figures





References
-
- Alpert P. Constraints of tolerance: why are desiccation-tolerant organisms so small or rare? Journal of Experimental Biology. 2006;209:1575–1584. - PubMed
-
- Arts T. Rhizoidal tubers and protonemal gemmae in European Ditrichum species. Journal of Bryology. 1994;18:43–61.
-
- Beckett RP. ABA-induced tolerance to ion leakage during rehydration following desiccation in the moss Atrichum androgynum. Plant Growth Regulation. 2001;35:131–135.
-
- Beckett RP, Csintalan Z, Tuba Z. ABA treatment increases both the desiccation tolerance of photosynthesis, and nonphotochemical quenching in the moss Atrichum undulatum. Plant Ecology. 2000;151:65–71.
-
- Benson EE. Cryopreservation. In: Benson EE, editor. Plant conservation biotechnology. London: Taylor & Francis; 1999. pp. 83–95.

