Intrinsic disorder in the C-terminal domain of the Shaker voltage-activated K+ channel modulates its interaction with scaffold proteins
- PMID: 17666528
- PMCID: PMC1941827
- DOI: 10.1073/pnas.0704059104
Intrinsic disorder in the C-terminal domain of the Shaker voltage-activated K+ channel modulates its interaction with scaffold proteins
Abstract
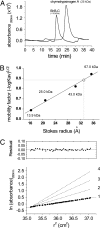
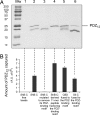
The interaction of membrane-embedded voltage-activated potassium channels (Kv) with intracellular scaffold proteins, such as the postsynaptic density 95 (PSD-95) protein, is mediated by the channel C-terminal segment. This interaction underlies Kv channel clustering at unique membrane sites and is important for the proper assembly and functioning of the synapse. In the current study, we address the molecular mechanism underlying Kv/PSD-95 interaction. We provide experimental evidence, based on hydrodynamic and spectroscopic analyses, indicating that the isolated C-terminal segment of the archetypical Shaker Kv channel (ShB-C) is a random coil, suggesting that ShB-C belongs to the recently defined class of intrinsically disordered proteins. We show that isolated ShB-C is still able to bind its scaffold protein partner and support protein clustering in vivo, indicating that unfoldedness is compatible with ShB-C activity. Pulldown experiments involving C-terminal chains differing in flexibility or length further demonstrate that intrinsic disorder in the C-terminal segment of the Shaker channel modulates its interaction with the PSD-95 protein. Our results thus suggest that the C-terminal domain of the Shaker Kv channel behaves as an entropic chain and support a "fishing rod" molecular mechanism for Kv channel binding to scaffold proteins. The importance of intrinsically disordered protein segments to the complex processes of synapse assembly, maintenance, and function is discussed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

