Validation of the SCID-hu Thy/Liv mouse model with four classes of licensed antiretrovirals
- PMID: 17668043
- PMCID: PMC1925140
- DOI: 10.1371/journal.pone.0000655
Validation of the SCID-hu Thy/Liv mouse model with four classes of licensed antiretrovirals
Abstract
Background: The SCID-hu Thy/Liv mouse model of HIV-1 infection is a useful platform for the preclinical evaluation of antiviral efficacy in vivo. We performed this study to validate the model with representatives of all four classes of licensed antiretrovirals.
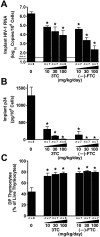
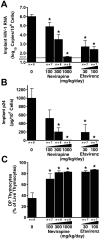
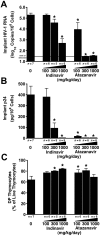
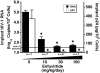
Methodology/principal findings: Endpoint analyses for quantification of Thy/Liv implant viral load included ELISA for cell-associated p24, branched DNA assay for HIV-1 RNA, and detection of infected thymocytes by intracellular staining for Gag-p24. Antiviral protection from HIV-1-mediated thymocyte depletion was assessed by multicolor flow cytometric analysis of thymocyte subpopulations based on surface expression of CD3, CD4, and CD8. These mice can be productively infected with molecular clones of HIV-1 (e.g., the X4 clone NL4-3) as well as with primary R5 and R5X4 isolates. To determine whether results in this model are concordant with those found in humans, we performed direct comparisons of two drugs in the same class, each of which has known potency and dosing levels in humans. Here we show that second-generation antiretrovirals were, as expected, more potent than their first-generation predecessors: emtricitabine was more potent than lamivudine, efavirenz was more potent than nevirapine, and atazanavir was more potent than indinavir. After interspecies pharmacodynamic scaling, the dose ranges found to inhibit viral replication in the SCID-hu Thy/Liv mouse were similar to those used in humans. Moreover, HIV-1 replication in these mice was genetically stable; treatment of the mice with lamivudine did not result in the M184V substitution in reverse transcriptase, and the multidrug-resistant NY index case HIV-1 retained its drug-resistance substitutions.
Conclusion: Given the fidelity of such comparisons, we conclude that this highly reproducible mouse model is likely to predict clinical antiviral efficacy in humans.
Conflict of interest statement
Figures



References
-
- McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, et al. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. - PubMed
-
- Namikawa R, Kaneshima H, Lieberman M, Weissman IL, McCune JM. Infection of the SCID-hu mouse by HIV-1. Science. 1988;242:1684–1686. - PubMed
-
- Kitchen SG, Zack JA. HIV type 1 infection in lymphoid tissue: natural history and model systems. AIDS Res Hum Retroviruses. 1998;14(Suppl 3):S235–239. - PubMed
-
- McCune JM. Development and applications of the SCID-hu mouse model. Semin Immunol. 1996;8:187–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

