Cathepsin K null mice show reduced adiposity during the rapid accumulation of fat stores
- PMID: 17668061
- PMCID: PMC1925145
- DOI: 10.1371/journal.pone.0000683
Cathepsin K null mice show reduced adiposity during the rapid accumulation of fat stores
Abstract
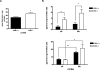
Growing evidences indicate that proteases are implicated in adipogenesis and in the onset of obesity. We previously reported that the cysteine protease cathepsin K (ctsk) is overexpressed in the white adipose tissue (WAT) of obese individuals. We herein characterized the WAT and the metabolic phenotype of ctsk deficient animals (ctsk-/-). When the growth rate of ctsk-/- was compared to that of the wild type animals (WT), we could establish a time window (5-8 weeks of age) within which ctsk-/-display significantly lower body weight and WAT size as compared to WT. Such a difference was not observable in older mice. Upon treatment with high fat diet (HFD) for 12 weeks ctsk-/- gained significantly less weight than WT and showed reduced brown adipose tissue, liver mass and a lower percentage of body fat. Plasma triglycerides, cholesterol and leptin were significantly lower in HFD-fed-ctsk-/- as compared to HFD-fed WT animals. Adipocyte lipolysis rates were increased in both young and HFD-fed-ctsk-/-, as compared to WT. Carnitine palmitoyl transferase-1 activity, was higher in mitochondria isolated from the WAT of HFD treated ctsk-/- as compared to WT. Together, these data indicate that ctsk ablation in mice results in reduced body fat content under conditions requiring a rapid accumulation of fat stores. This observation could be partly explained by an increased release and/or utilization of FFA and by an augmented ratio of lipolysis/lipogenesis. These results also demonstrate that under a HFD, ctsk deficiency confers a partial resistance to the development of dyslipidemia.
Conflict of interest statement
Figures



Similar articles
-
Decreased lipogenesis in white adipose tissue contributes to the resistance to high fat diet-induced obesity in phosphatidylethanolamine N-methyltransferase-deficient mice.Biochim Biophys Acta. 2015 Feb;1851(2):152-62. doi: 10.1016/j.bbalip.2014.11.006. Epub 2014 Nov 15. Biochim Biophys Acta. 2015. PMID: 25463480
-
Regulatory role of metallothionein-1/2 on development of sex differences in a high-fat diet-induced obesity.Life Sci. 2019 Jun 1;226:12-21. doi: 10.1016/j.lfs.2019.04.012. Epub 2019 Apr 4. Life Sci. 2019. PMID: 30954474
-
miR-155 Deletion in Female Mice Prevents Diet-Induced Obesity.Sci Rep. 2016 Mar 8;6:22862. doi: 10.1038/srep22862. Sci Rep. 2016. PMID: 26953132 Free PMC article.
-
Adipocyte Septin-7 attenuates obesogenic adipogenesis and promotes lipolysis to prevent obesity.Mol Metab. 2025 May;95:102114. doi: 10.1016/j.molmet.2025.102114. Epub 2025 Feb 25. Mol Metab. 2025. PMID: 40015624 Free PMC article.
-
Identification of cathepsin K as a novel marker of adiposity in white adipose tissue.J Cell Physiol. 2003 May;195(2):309-21. doi: 10.1002/jcp.10253. J Cell Physiol. 2003. PMID: 12652657
Cited by
-
Haptoglobin deficiency determines changes in adipocyte size and adipogenesis.Adipocyte. 2012 Jul 1;1(3):142-183. doi: 10.4161/adip.20041. Adipocyte. 2012. PMID: 23700523 Free PMC article.
-
Cathepsin K inhibition promotes efficient differentiation of human embryonic stem cells to mature cardiomyocytes by mediating glucolipid metabolism and cellular energy homeostasis.Stem Cell Res Ther. 2025 Mar 5;16(1):118. doi: 10.1186/s13287-025-04231-7. Stem Cell Res Ther. 2025. PMID: 40045408 Free PMC article.
-
ECM roles in the function of metabolic tissues.Trends Endocrinol Metab. 2012 Jan;23(1):16-22. doi: 10.1016/j.tem.2011.09.006. Epub 2011 Nov 8. Trends Endocrinol Metab. 2012. PMID: 22070921 Free PMC article. Review.
-
A single nucleotide polymorphism in the porcine cathepsin K (CTSK) gene is associated with back fat thickness and production traits in Italian Duroc pigs.Mol Biol Rep. 2010 Jan;37(1):491-5. doi: 10.1007/s11033-009-9678-0. Epub 2009 Aug 7. Mol Biol Rep. 2010. PMID: 19662513
-
A sensitive period for environmental regulation of eating behavior and leptin sensitivity.Proc Natl Acad Sci U S A. 2010 Sep 21;107(38):16673-8. doi: 10.1073/pnas.0911832107. Epub 2010 Sep 7. Proc Natl Acad Sci U S A. 2010. PMID: 20823242 Free PMC article.
References
-
- Lecaille F, Choe Y, Brandt W, Li Z, Craik CS, et al. Selective inhibition of the collagenolytic activity of human cathepsin K by altering its S2 subsite specificity. Biochemistry. 2002;41:8447–8454. - PubMed
-
- Turk D, Turk B, Turk V. Papain-like lysosomal cysteine proteases and their inhibitors: drug discovery targets? Biochem Soc Symp. 2003:15–30. - PubMed
-
- Yasuda Y, Kaleta J, Bromme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev. 2005;57:973–993. - PubMed
-
- Buhling F, Reisenauer A, Gerber A, Kruger S, Weber E, et al. Cathepsin K–a marker of macrophage differentiation? J Pathol. 2001;195:375–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

